The LIM homeodomain protein Lhx6 regulates maturation of interneurons and network excitability in the mammalian cortex
- PMID: 22710612
- PMCID: PMC3698365
- DOI: 10.1093/cercor/bhs159
The LIM homeodomain protein Lhx6 regulates maturation of interneurons and network excitability in the mammalian cortex
Abstract
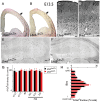
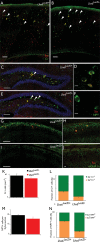
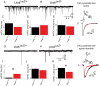
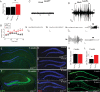
Deletion of LIM homeodomain transcription factor-encoding Lhx6 gene in mice results in defective tangential migration of cortical interneurons and failure of differentiation of the somatostatin (Sst)- and parvalbumin (Pva)-expressing subtypes. Here, we characterize a novel hypomorphic allele of Lhx6 and demonstrate that reduced activity of this locus leads to widespread differentiation defects in Sst(+) interneurons, but relatively minor and localized changes in Pva(+) interneurons. The reduction in the number of Sst-expressing cells was not associated with a loss of interneurons, because the migration and number of Lhx6-expressing interneurons and expression of characteristic molecular markers, such as calretinin or Neuropeptide Y, were not affected in Lhx6 hypomorphic mice. Consistent with a selective deficit in the differentiation of Sst(+) interneurons in the CA1 subfield of the hippocampus, we observed reduced expression of metabotropic Glutamate Receptor 1 in the stratum oriens and characteristic changes in dendritic inhibition, but normal inhibitory input onto the somatic compartment of CA1 pyramidal cells. Moreover, Lhx6 hypomorphs show behavioral, histological, and electroencephalographic signs of recurrent seizure activity, starting from early adulthood. These results demonstrate that Lhx6 plays an important role in the maturation of cortical interneurons and the formation of inhibitory circuits in the mammalian cortex.
Keywords: LIM homeodomain protein; Lhx6; cortical interneurons.
Figures




Similar articles
-
Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes.J Neurosci. 2007 Mar 21;27(12):3078-89. doi: 10.1523/JNEUROSCI.3055-06.2007. J Neurosci. 2007. PMID: 17376969 Free PMC article.
-
CTCF Governs the Identity and Migration of MGE-Derived Cortical Interneurons.J Neurosci. 2019 Jan 2;39(1):177-192. doi: 10.1523/JNEUROSCI.3496-17.2018. Epub 2018 Oct 30. J Neurosci. 2019. PMID: 30377227 Free PMC article.
-
Distinct molecular pathways for development of telencephalic interneuron subtypes revealed through analysis of Lhx6 mutants.J Comp Neurol. 2008 Sep 1;510(1):79-99. doi: 10.1002/cne.21772. J Comp Neurol. 2008. PMID: 18613121 Free PMC article.
-
The development of MGE-derived cortical interneurons: An Lhx6 tale.Int J Dev Biol. 2022;66(1-2-3):43-49. doi: 10.1387/ijdb.210185md. Int J Dev Biol. 2022. PMID: 34881792 Review.
-
Lhx6 and Lhx8: cell fate regulators and beyond.FASEB J. 2015 Oct;29(10):4083-91. doi: 10.1096/fj.14-267500. Epub 2015 Jul 6. FASEB J. 2015. PMID: 26148970 Free PMC article. Review.
Cited by
-
Interneuron function and cognitive behavior are preserved upon postnatal removal of Lhx6.Sci Rep. 2022 Mar 22;12(1):4923. doi: 10.1038/s41598-022-09003-4. Sci Rep. 2022. PMID: 35318414 Free PMC article.
-
Nf1 deletion results in depletion of the Lhx6 transcription factor and a specific loss of parvalbumin+ cortical interneurons.Proc Natl Acad Sci U S A. 2020 Mar 17;117(11):6189-6195. doi: 10.1073/pnas.1915458117. Epub 2020 Mar 2. Proc Natl Acad Sci U S A. 2020. PMID: 32123116 Free PMC article.
-
Altered expression of developmental regulators of parvalbumin and somatostatin neurons in the prefrontal cortex in schizophrenia.Schizophr Res. 2016 Nov;177(1-3):3-9. doi: 10.1016/j.schres.2016.03.001. Epub 2016 Mar 10. Schizophr Res. 2016. PMID: 26972474 Free PMC article.
-
Genetic and activity-dependent mechanisms underlying interneuron diversity.Nat Rev Neurosci. 2017 May;18(5):299-309. doi: 10.1038/nrn.2017.30. Epub 2017 Apr 6. Nat Rev Neurosci. 2017. PMID: 28381833 Review.
-
Involvement of cortical fast-spiking parvalbumin-positive basket cells in epilepsy.Prog Brain Res. 2016;226:81-126. doi: 10.1016/bs.pbr.2016.04.012. Epub 2016 Jun 7. Prog Brain Res. 2016. PMID: 27323940 Free PMC article. Review.
References
-
- Ali AB, Thomson AM. Synaptic alpha 5 subunit-containing GABAA receptors mediate IPSPs elicited by dendrite-preferring cells in rat neocortex. Cereb Cortex. 2008;18:1260–1271. doi:10.1093/cercor/bhm160. - DOI - PubMed
-
- Alifragis P, Liapi A, Parnavelas JG. Lhx6 regulates the migration of cortical interneurons from the ventral telencephalon but does not specify their GABA phenotype. J Neurosci. 2004;24:5643–5648. doi:10.1523/JNEUROSCI.1245-04.2004. - DOI - PMC - PubMed
-
- Andersen P, Eccles JC, Loyning Y. Recurrent inhibition in the hippocampus with identification of the inhibitory cell and its synapses. Nature. 1963;198:540–542. doi:10.1038/198540a0. - DOI - PubMed
-
- Andrasfalvy BK, Mody I. Differences between the scaling of miniature IPSCs and EPSCs recorded in the dendrites of CA1 mouse pyramidal neurons. J Physiol. 2006;576:191–196. doi:10.1113/jphysiol.2006.115428. - DOI - PMC - PubMed
-
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsaki G, Cauli B, Defelipe J, Fairen A, et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–568. doi:10.1038/nrn2402. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

