Age-based comparison of human dendritic spine structure using complete three-dimensional reconstructions
- PMID: 22710613
- PMCID: PMC3698364
- DOI: 10.1093/cercor/bhs154
Age-based comparison of human dendritic spine structure using complete three-dimensional reconstructions
Abstract
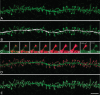
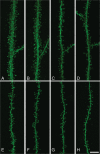
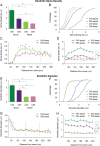
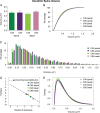
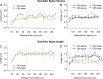
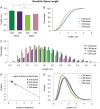
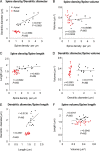
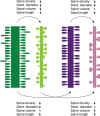
Dendritic spines of pyramidal neurons are targets of most excitatory synapses in the cerebral cortex. Recent evidence suggests that the morphology of the dendritic spine could determine its synaptic strength and learning rules. However, unfortunately, there are scant data available regarding the detailed morphology of these structures for the human cerebral cortex. In the present study, we analyzed over 8900 individual dendritic spines that were completely 3D reconstructed along the length of apical and basal dendrites of layer III pyramidal neurons in the cingulate cortex of 2 male humans (aged 40 and 85 years old), using intracellular injections of Lucifer Yellow in fixed tissue. We assembled a large, quantitative database, which revealed a major reduction in spine densities in the aged case. Specifically, small and short spines of basal dendrites and long spines of apical dendrites were lost, regardless of the distance from the soma. Given the age difference between the cases, our results suggest selective alterations in spines with aging in humans and indicate that the spine volume and length are regulated by different biological mechanisms.
Keywords: 3D reconstructions; Lucifer Yellow; cerebral cortex; confocal; intracellular.
Figures

References
-
- Anderson K, Bones B, Robinson B, Hass C, Lee H, Ford K, Roberts TA, Jacobs B. The morphology of supragranular pyramidal neurons in the human insular cortex: a quantitative Golgi study. Cerebral Cortex. 2009;19:2131–2144. - PubMed
-
- Arellano JI, Espinosa A, Fairén A, Yuste R, DeFelipe J. Non-synaptic dendritic spines in neocortex. Neuroscience. 2007;145:464–469. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

