TGF-β stimulates Pyk2 expression as part of an epithelial-mesenchymal transition program required for metastatic outgrowth of breast cancer
- PMID: 22710711
- PMCID: PMC3721373
- DOI: 10.1038/onc.2012.230
TGF-β stimulates Pyk2 expression as part of an epithelial-mesenchymal transition program required for metastatic outgrowth of breast cancer
Abstract
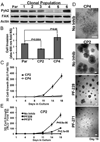
Epithelial-mesenchymal transition (EMT) programs are essential in promoting breast cancer invasion, systemic dissemination and in arousing proliferative programs in breast cancer micrometastases, a reaction that is partially dependent on focal adhesion kinase (FAK). Many functions of FAK are shared by its homolog, protein tyrosine kinase 2 (Pyk2), raising the question as to whether Pyk2 also participates in driving the metastatic outgrowth of disseminated breast cancer cells. In addressing this question, we observed Pyk2 expression to be (i) significantly upregulated in recurrent human breast cancers; (ii) differentially expressed across clonal isolates of human MDA-MB-231 breast cancer cells in a manner predictive for metastatic outgrowth, but not for invasiveness; and (iii) dramatically elevated in ex vivo cultures of breast cancer cells isolated from metastatic lesions as compared with cells that produced the primary tumor. We further show that metastatic human and murine breast cancer cells robustly upregulate their expression of Pyk2 during EMT programs stimulated by transforming growth factor-β (TGF-β). Genetic and pharmacological inhibition of Pyk2 demonstrated that the activity of this protein tyrosine kinase was dispensable for the ability of breast cancer cells to undergo invasion in response to TGF-β, and to form orthotopic mammary tumors in mice. In stark contrast, Pyk2-deficiency prevented TGF-β from stimulating the growth of breast cancer cells in 3D-organotypic cultures that recapitulated pulmonary microenvironments, as well as inhibited the metastatic outgrowth of disseminated breast cancer cells in the lungs of mice. Mechanistically, Pyk2 expression was inversely related to that of E-cadherin, such that elevated Pyk2 levels stabilized β1 integrin expression necessary to initiate the metastatic outgrowth of breast cancer cells. Thus, we have delineated novel functions for Pyk2 in mediating distinct elements of the EMT program and metastatic cascade regulated by TGF-β, particularly the initiation of secondary tumor outgrowth by disseminated cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


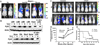




Similar articles
-
Therapeutic targeting of the focal adhesion complex prevents oncogenic TGF-beta signaling and metastasis.Breast Cancer Res. 2009;11(5):R68. doi: 10.1186/bcr2360. Breast Cancer Res. 2009. PMID: 19740433 Free PMC article.
-
Fibroblast growth factor receptor splice variants are stable markers of oncogenic transforming growth factor β1 signaling in metastatic breast cancers.Breast Cancer Res. 2014 Mar 11;16(2):R24. doi: 10.1186/bcr3623. Breast Cancer Res. 2014. PMID: 24618085 Free PMC article.
-
Down-regulation of epithelial cadherin is required to initiate metastatic outgrowth of breast cancer.Mol Biol Cell. 2011 Jul 15;22(14):2423-35. doi: 10.1091/mbc.E11-04-0306. Epub 2011 May 25. Mol Biol Cell. 2011. PMID: 21613543 Free PMC article.
-
The pathophysiology of epithelial-mesenchymal transition induced by transforming growth factor-beta in normal and malignant mammary epithelial cells.J Mammary Gland Biol Neoplasia. 2010 Jun;15(2):169-90. doi: 10.1007/s10911-010-9181-1. Epub 2010 May 15. J Mammary Gland Biol Neoplasia. 2010. PMID: 20467795 Free PMC article. Review.
-
Noncanonical TGF-β signaling during mammary tumorigenesis.J Mammary Gland Biol Neoplasia. 2011 Jun;16(2):127-46. doi: 10.1007/s10911-011-9207-3. Epub 2011 Mar 31. J Mammary Gland Biol Neoplasia. 2011. PMID: 21448580 Free PMC article. Review.
Cited by
-
Targeting the Metabolic Reprogramming That Controls Epithelial-to-Mesenchymal Transition in Aggressive Tumors.Front Oncol. 2017 Mar 14;7:40. doi: 10.3389/fonc.2017.00040. eCollection 2017. Front Oncol. 2017. PMID: 28352611 Free PMC article. Review.
-
Mechanisms of TGFβ-Induced Epithelial-Mesenchymal Transition.J Clin Med. 2016 Jun 29;5(7):63. doi: 10.3390/jcm5070063. J Clin Med. 2016. PMID: 27367735 Free PMC article. Review.
-
ZNF488 Enhances the Invasion and Tumorigenesis in Nasopharyngeal Carcinoma Via the Wnt Signaling Pathway Involving Epithelial Mesenchymal Transition.Cancer Res Treat. 2016 Jan;48(1):334-44. doi: 10.4143/crt.2014.311. Epub 2015 Mar 12. Cancer Res Treat. 2016. PMID: 25779368 Free PMC article.
-
Transcriptomic Analysis of Subtype-Specific Tyrosine Kinases as Triple Negative Breast Cancer Biomarkers.Cancers (Basel). 2023 Jan 7;15(2):403. doi: 10.3390/cancers15020403. Cancers (Basel). 2023. PMID: 36672350 Free PMC article.
-
Fibroblast growth receptor 1 is regulated by G-quadruplex in metastatic breast cancer.Commun Biol. 2024 Aug 9;7(1):963. doi: 10.1038/s42003-024-06602-x. Commun Biol. 2024. PMID: 39122837 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

