The ubiquitin-specific protease USP2a prevents endocytosis-mediated EGFR degradation
- PMID: 22710717
- PMCID: PMC3866888
- DOI: 10.1038/onc.2012.188
The ubiquitin-specific protease USP2a prevents endocytosis-mediated EGFR degradation
Abstract
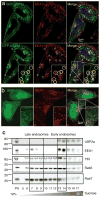
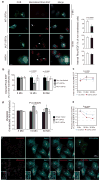
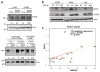
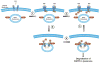
Ubiquitination of epidermal growth factor receptor (EGFR) is required for downregulation of the receptor by endocytosis. Impairment of this pathway results in constitutively active EGFR, which is associated with carcinogenesis, particularly in lung cancer. We previously demonstrated that the deubiquitinating enzyme ubiquitin-specific protease 2a (USP2a) has oncogenic properties. Here, we show a new role for USP2a as a regulator of EGFR endocytosis. USP2a localizes to early endosomes and associates with EGFR, stabilizing the receptor, which retains active downstream signaling. HeLa cells transiently expressing catalytically active, but not mutant (MUT), USP2a show increased plasma membrane-localized EGFR, as well as decreased internalized and ubiquitinated EGFR. Conversely, USP2a silencing reverses this phenotype. Importantly, USP2a prevents the degradation of MUT in addition to wild-type EGFR. Finally, we observed that USP2a and EGFR proteins are coordinately overexpressed in non-small cell lung cancers. Taken together, our data indicate that USP2a antagonizes EGFR endocytosis and thus amplifies signaling activity from the receptor. Our findings suggest that regulation of deubiquitination could be exploited therapeutically in cancers overexpressing EGFR.
Figures

References
-
- Sorkin A, Von Zastrow M. Signal transduction and endocytosis: close encounters of many kinds. Nat Rev Mol Cell Biol. 2002;3:600–614. - PubMed
-
- Haglund K, Sigismund S, Polo S, Szymkiewicz I, Di Fiore PP, Dikic I. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat Cell Biol. 2003;5:461–466. - PubMed
-
- Mosesson Y, Shtiegman K, Katz M, Zwang Y, Vereb G, Szollosi J, et al. Endocytosis of receptor tyrosine kinases is driven by monoubiquitylation, not polyubiquitylation. J Biol Chem. 2003;278:21323–21326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

