Angiotensin-(1-7) via the mas receptor alleviates the diabetes-induced decrease in GFAP and GAP-43 immunoreactivity with concomitant reduction in the COX-2 in hippocampal formation: an immunohistochemical study
- PMID: 22711212
- PMCID: PMC11498388
- DOI: 10.1007/s10571-012-9858-7
Angiotensin-(1-7) via the mas receptor alleviates the diabetes-induced decrease in GFAP and GAP-43 immunoreactivity with concomitant reduction in the COX-2 in hippocampal formation: an immunohistochemical study
Abstract
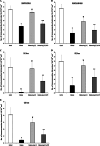
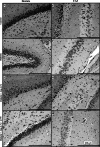
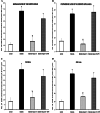
We have previously shown that chronic treatment with angiotensin-(1-7) [Ang-(1-7)] can prevent diabetes-induced cardiovascular dysfunction. However, effect of Ang-(1-7) treatment on diabetes-induced alterations in the CNS is unknown. The aim of this study was to test the hypothesis that treatment with Ang-(1-7) can produce protection against diabetes-induced CNS changes. We examined the effect of Ang-(1-7) on the number of cyclooxygenase-2 (COX-2) immunoreactive neurons and the glial fibrillary acidic protein (GFAP)-immunoreactive astrocytes and assessed the changes in the neuronal growth-associated protein-43 (GAP-43) of the hippocampal formation in streptozotocin-induced diabetes in rats. Animals were sacrificed 30 days after induction of diabetes and/or treatment with Ang-(1-7). Ang-(1-7) treatment significantly prevented diabetes-induced decrease in the number of GFAP immunoreactive astrocytes and GAP-43 positive neurons in all hippocampal regions. Co-administration of A779, a selective Ang-(1-7) receptor antagonist, inhibited Ang-(1-7)-mediated protective effects indicating that Ang-(1-7) produces its effects through activation of receptor Mas. Further, Ang-(1-7) treatment through activation of Mas significantly prevented diabetes-induced increase in the number of the COX-2 immunolabeled neurons in all sub-regions of the hippocampus examined. These results show that Ang-(1-7) has a protective role against diabetes-induced changes in the CNS.
Figures



References
-
- Afsari ZH, Renno WM, Abd-El-Basset E (2008) Alteration of glial fibrillary acidic proteins immunoreactivity in astrocytes of the spinal cord diabetic rats. Anat Rec 291:390–399 - PubMed
-
- Artola A, Kamal A, Ramakers GM, Biessels GJ, Gispen WH (2005) Diabetes mellitus concomitantly facilitates the induction of long-term depression and inhibits that of long-term potentiation in hippocampus. Euro J Neurosci 22:169–178 - PubMed
-
- Bagi Z, Erdei N, Papp Z, Edes I, Koller A (2006) Up-regulation of vascular cyclooxygenase-2 in diabetes mellitus. Pharmacol Rep 58:52–56 - PubMed
-
- Barber AJ, Antonetti DA, Gardner TW (2000) Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. Investig Ophthalmol Vis Sci 41:3561–3568 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

