Correlating cellulose derivative intrinsic viscosity with mechanical susceptibility of swollen hydrophilic matrix tablets
- PMID: 22711256
- PMCID: PMC3429674
- DOI: 10.1208/s12249-012-9811-6
Correlating cellulose derivative intrinsic viscosity with mechanical susceptibility of swollen hydrophilic matrix tablets
Abstract
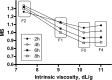
Hydrophilic matrix tablets are prone to mechanical stress while passing through the gastrointestinal tract, which may result in inappropriate drug-release characteristics. Intrinsic viscosity is a physical polymer property that can be directly compared across various types and grades of polymers and correlated with the mechanical susceptibility of swollen matrix tablets. Five tablet formulations containing different HPMC and HPC polymers were prepared and analyzed using an in vitro glass bead manipulation test. The dissolution rate results were modeled using the Korsmeyer-Peppas equation and a correlation was found between the fit constants k and n, goodness-of-fit measure parameters, and intrinsic viscosity. Moreover, the dissolution profiles were used to calculate the degree of mechanical susceptibility for each formulation, defined as the ratio of the average dissolution rate after manipulation and the initial dissolution rate before manipulation. It was confirmed that an increased intrinsic viscosity polymer value resulted in a decrease in mechanical susceptibility. Considering this, two simple rules were defined for designing robust matrix tablets with respect to mechanical stresses.
Figures






References
-
- Liu P, Ju T, Qiu Y. Diffusion-controlled drug delivery systems. In: Li X, Jasti BR, editors. Design of controlled release drug delivery systems. United States: McGraw-Hill; 2006. pp. 107–137.
-
- Wang Z, Shmeis RA. Dissolution controlled drug delivery systems. In: Li X, Jasti BR, editors. Design of controlled release drug delivery systems. United States: McGraw-Hill; 2006. pp. 139–172.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

