Histone deacetylation inhibition in pulmonary hypertension: therapeutic potential of valproic acid and suberoylanilide hydroxamic acid
- PMID: 22711276
- PMCID: PMC3799888
- DOI: 10.1161/CIRCULATIONAHA.112.103176
Histone deacetylation inhibition in pulmonary hypertension: therapeutic potential of valproic acid and suberoylanilide hydroxamic acid
Abstract
Background: Epigenetic programming, dynamically regulated by histone acetylation, is a key mechanism regulating cell proliferation and survival. Little is known about the contribution of histone deacetylase (HDAC) activity to the development of pulmonary arterial hypertension, a condition characterized by profound structural remodeling of pulmonary arteries and arterioles.
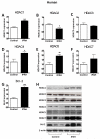
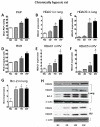
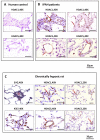
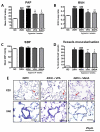
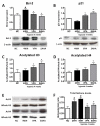
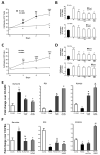
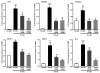
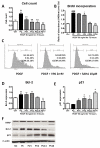
Methods and results: HDAC1 and HDAC5 protein levels were elevated in lungs from human idiopathic pulmonary arterial hypertension and in lungs and right ventricles from rats exposed to hypoxia. Immunohistochemistry localized increased expression to remodeled vessels in the lung. Both valproic acid, a class I HDAC inhibitor, and suberoylanilide hydroxamic acid (vorinostat), an inhibitor of class I, II, and IV HDACs, mitigated the development of and reduced established hypoxia-induced pulmonary hypertension in the rat. Both valproic acid and suberoylanilide hydroxamic acid inhibited the imprinted highly proliferative phenotype of fibroblasts and R-cells from pulmonary hypertensive bovine vessels and platelet-derived growth factor-stimulated growth of human vascular smooth muscle cells in culture. Exposure to valproic acid and suberoylanilide hydroxamic acid was associated with increased levels of p21 and FOXO3 and reduced expression of survivin. The significantly higher levels of expression of cKIT, monocyte chemoattractant protein-1, interleukin-6, stromal-derived factor-1, platelet-derived growth factor-b, and S100A4 in R-cells were downregulated by valproic acid and suberoylanilide hydroxamic acid treatment.
Conclusions: Increased HDAC activity contributes to the vascular pathology of pulmonary hypertension. The effectiveness of HDAC inhibitors, valproic acid, and suberoylanilide hydroxamic acid, in models of pulmonary arterial hypertension supports a therapeutic strategy based on HDAC inhibition in pulmonary arterial hypertension.
Figures
Comment in
-
Letter by Bogaard et al regarding article, "histone deacetylation inhibition in pulmonary hypertension: therapeutic potential of valproic acid and suberoylanilide hydroxamic acid".Circulation. 2013 Apr 9;127(14):e539. doi: 10.1161/CIRCULATIONAHA.112.127092. Circulation. 2013. PMID: 23569123 No abstract available.
-
Response to letter regarding article, “histone deacetylation inhibition in pulmonary hypertension: therapeutic potential of valproic acid and suberoylanilide hydroxamic acid”.Circulation. 2013 Apr 9;127(14):e540. doi: 10.1161/circulationaha.112.154757. Circulation. 2013. PMID: 23691553 No abstract available.
References
-
- Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, Newman JH, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel NF, Yuan JX, Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54:S10–S19. - PubMed
-
- Tuder RM, Abman SH, Braun T, Capron F, Stevens T, Thistlethwaite PA, Haworth SG. Development and pathology of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S3–S9. - PubMed
-
- Richards EJ, Elgin SC. Epigenetic codes for heterochromatin formation and silencing: rounding up the usual suspects. Cell. 2002;108:489–500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

