Biomarkers and surrogate endpoints in clinical trials
- PMID: 22711298
- PMCID: PMC3551627
- DOI: 10.1002/sim.5403
Biomarkers and surrogate endpoints in clinical trials
Abstract
One of the most important considerations in designing clinical trials is the choice of outcome measures. These outcome measures could be clinically meaningful endpoints that are direct measures of how patients feel, function, and survive. Alternatively, indirect measures, such as biomarkers that include physical signs of disease, laboratory measures, and radiological tests, often are considered as replacement endpoints or 'surrogates' for clinically meaningful endpoints. We discuss the definitions of clinically meaningful endpoints and surrogate endpoints, and provide examples from recent clinical trials. We provide insight into why indirect measures such as biomarkers may fail to provide reliable evidence about the benefit-to-risk profile of interventions. We also discuss the nature of evidence that is important in assessing whether treatment effects on a biomarker reliably predict effects on a clinically meaningful endpoint, and provide insights into why this reliability is specific to the context of use of the biomarker.
Copyright © 2012 John Wiley & Sons, Ltd.
Figures



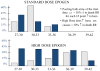
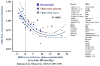
References
-
- US Government Printing Office. Applications for FDA approval to market a new drug: adequate and well-controlled studies. [Accessed November 29, 2011.];US Code title 21, section 314.126. Available at: http://edocket.access.gpo.gov/cfr_2009/aprqtr/21cfr314.126.htm.
-
- Patrick DL, Burke LB, Gwaltney CJ, Leidy NK, Martin ML, Molsen E, Ring L. [Accessed November 29, 2011.];Content Validity—Establishing and Reporting the Evidence in Newly Developed Patient-Reported Outcomes (PRO) Instruments for Medical Product Evaluation: ISPOR PRO Good Research Practices Task Force Report: Part 1—Eliciting Concepts for a New PRO Instrument. Available at: http://www.valueinhealthjournal.com/article/S1098-3015(11)03323-7/abstract. - PubMed
-
- Patrick DL, Burke LB, Gwaltney CJ, Leidy NK, Martin ML, Molsen E, Ring L. [Accessed November 29, 2011.];Content Validity—Establishing and Reporting the Evidence in Newly Developed Patient-Reported Outcomes (PRO) Instruments for Medical Product Evaluation: ISPOR PRO Good Research Practices Task Force Report: Part 2—Assessing Respondent Understanding. Available at: http://www.valueinhealthjournal.com/article/S1098-3015(11)03321-3/abstract. - PubMed
-
- Guidance for Industry Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), Center for Devices and Radiological Health (CDRH); Dec, 2009. [Accessed November 29, 2011.]. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformati....
-
- Dormandy JA, Charbonnel B, Eckland EJA, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes: a randomized trial of pioglitazone. The PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events) Lancet. 2005;366:1279–1289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
