Clinical and molecular characteristics of congenital glioblastoma
- PMID: 22711608
- PMCID: PMC3379807
- DOI: 10.1093/neuonc/nos125
Clinical and molecular characteristics of congenital glioblastoma
Abstract
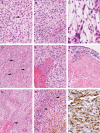
Congenital glioblastoma (cGBM) is an uncommon tumor of infancy with a reported variable but often poor cure rate, even with intensive therapy. Five patients with cGBMs, arising de novo and not in familial tumor predisposition kindreds, were studied for histological and biological features, using Affymetrix microarray. Tumors were large, often associated with hemorrhage, extended into the thalamus, and often bulged into the ventricles. One patient died acutely from bleeding at the time of operation. The 4 surviving patients underwent surgery (1 gross total resection, 3 subtotal resections or biopsies) and moderate intensity chemotherapy without radiation, and remain progression-free at a median time of 36 months (range, 30-110 months). Affymetrix microarrays measured gene expression on the 3 cGBMs from which frozen tissue was available. Unsupervised hierarchical clustering of cGBMs versus 168 other central nervous system tumors demonstrated that cGBMs clustered most closely with other high-grade gliomas. Gene expression profiles of cGBMs were compared with non-congenital pediatric and adult GBMs. cGBMs demonstrated marked similarity to both pediatric and adult GBMs, with only 31 differentially expressed genes identified (false discovery rate, <0.05). Unique molecular features of cGBMs included over-expression of multiple genes involved in glucose metabolism and tissue hypoxia. cGBMs show histological and biological overlap with pediatric and adult GBMs but appear to have a more favorable outcome, with good response to moderate intensity chemotherapy with only subtotal resection or biopsy. Further study may determine whether identified gene expression differences contribute to the improved survival seen in these tumors.
Figures



References
-
- Severino M, Schwartz ES, Thurnher MM, Rydland J, Nikas I, Rossi A. Congenital tumors of the central nervous system. Neuroradiology. 2010;52(6):531–548. - PubMed
-
- Mazewski CM, Hudgins RJ, Reisner A, Geyer JR. Neonatal brain tumors: a review. Semin Perinatol. 1999;23(4):286–298. - PubMed
-
- Rickert CH, Probst-Cousin S, Gullotta F. Primary intracranial neoplasms of infancy and early childhood. Childs Nerv Syst. 1997;13(10):507–513. - PubMed
-
- Jellinger K, Sunder-Plassmann M. Connatal intracranial tumours. Neuropadiatrie. 1973;4(1):46–63. - PubMed
-
- Manoranjan B, Provias JP. Congenital brain tumors: diagnostic pitfalls and therapeutic interventions. J Child Neurol. 2011;26(5):599–614. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

