Meiotic cohesin complexes are essential for the formation of the axial element in mice
- PMID: 22711701
- PMCID: PMC3384418
- DOI: 10.1083/jcb.201201100
Meiotic cohesin complexes are essential for the formation of the axial element in mice
Abstract
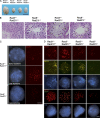
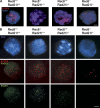
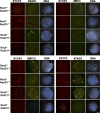
Cohesin is a conserved multisubunit protein complex that participates in chromosome segregation, DNA damage repair, chromatin regulation, and synaptonemal complex (SC) formation. Yeast, but not mice, depleted of the cohesin subunit Rec8 are defective in the formation of the axial elements (AEs) of the SC, suggesting that, in mammals, this function is not conserved. In this paper, we show that spermatocytes from mice lacking the two meiosis-specific cohesin subunits RAD21L and REC8 were unable to initiate RAD51- but not DMC1-mediated double-strand break repair, were not able to assemble their AEs, and arrested as early as the leptotene stage of prophase I, demonstrating that cohesin plays an essential role in AE assembly that is conserved from yeast to mammals.
Figures


References
-
- de Vries F.A., de Boer E., van den Bosch M., Baarends W.M., Ooms M., Yuan L., Liu J.G., van Zeeland A.A., Heyting C., Pastink A. 2005. Mouse Sycp1 functions in synaptonemal complex assembly, meiotic recombination, and XY body formation. Genes Dev. 19:1376–1389 10.1101/gad.329705 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

