The role of aldehyde oxidase and xanthine oxidase in the biotransformation of a novel negative allosteric modulator of metabotropic glutamate receptor subtype 5
- PMID: 22711749
- PMCID: PMC3422546
- DOI: 10.1124/dmd.112.046136
The role of aldehyde oxidase and xanthine oxidase in the biotransformation of a novel negative allosteric modulator of metabotropic glutamate receptor subtype 5
Abstract
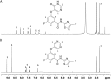
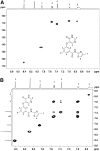
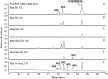
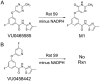
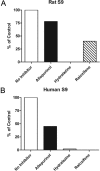
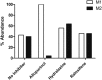
Negative allosteric modulation (NAM) of metabotropic glutamate receptor subtype 5 (mGlu₅) represents a therapeutic strategy for the treatment of childhood developmental disorders, such as fragile X syndrome and autism. VU0409106 emerged as a lead compound within a biaryl ether series, displaying potent and selective inhibition of mGlu₅. Despite its high clearance and short half-life, VU0409106 demonstrated efficacy in rodent models of anxiety after extravascular administration. However, lack of a consistent correlation in rat between in vitro hepatic clearance and in vivo plasma clearance for the biaryl ether series prompted an investigation into the biotransformation of VU0409106 using hepatic subcellular fractions. An in vitro appraisal in rat, monkey, and human liver S9 fractions indicated that the principal pathway was NADPH-independent oxidation to metabolite M1 (+16 Da). Both raloxifene (aldehyde oxidase inhibitor) and allopurinol (xanthine oxidase inhibitor) attenuated the formation of M1, thus implicating the contribution of both molybdenum hydroxylases in the biotransformation of VU0409106. The use of ¹⁸O-labeled water in the S9 experiments confirmed the hydroxylase mechanism proposed, because ¹⁸O was incorporated into M1 (+18 Da) as well as in a secondary metabolite (M2; +36 Da), the formation of which was exclusively xanthine oxidase-mediated. This unusual dual and sequential hydroxylase metabolism was confirmed in liver S9 and hepatocytes of multiple species and correlated with in vivo data because M1 and M2 were the principal metabolites detected in rats administered VU0409106. An in vitro-in vivo correlation of predicted hepatic and plasma clearance was subsequently established for VU0409106 in rats and nonhuman primates.
Figures


References
-
- Akabane T, Tanaka K, Irie M, Terashita S, Teramura T. (2011) Case report of extensive metabolism by aldehyde oxidase in humans: pharmacokinetics and metabolite profile of FK3453 in rats, dogs, and humans. Xenobiotica 41:372–384 - PubMed
-
- Balani SK, Miwa GT, Gan LS, Wu JT, Lee FW. (2005) Strategy of utilizing in vitro and in vivo ADME tools for lead optimization and drug candidate selection. Curr Top Med Chem 5:1033–1038 - PubMed
-
- Bear MF, Huber KM, Warren ST. (2004) The mGluR theory of fragile X mental retardation. Trends Neurosci 27:370–377 - PubMed
-
- Beedham C, Miceli JJ, Obach RS. (2003) Ziprasidone metabolism, aldehyde oxidase, and clinical implications. J Clin Psychopharmacol 23:229–232 - PubMed
-
- Dalvie D, Zhang C, Chen W, Smolarek T, Obach RS, Loi CM. (2010) Cross-species comparison of the metabolism and excretion of zoniporide: contribution of aldehyde oxidase to interspecies differences. Drug Metab Dispos 38:641–654 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information

