Crown ether-electrolyte interactions permit nanopore detection of individual DNA abasic sites in single molecules
- PMID: 22711805
- PMCID: PMC3406832
- DOI: 10.1073/pnas.1201669109
Crown ether-electrolyte interactions permit nanopore detection of individual DNA abasic sites in single molecules
Abstract
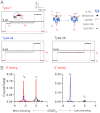
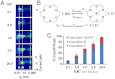
DNA abasic (AP) sites are one of the most frequent lesions in the genome and have a high mutagenic potential if unrepaired. After selective attachment of 2-aminomethyl-18-crown-6 (18c6), individual AP lesions are detected during electrophoretic translocation through the bacterial protein ion channel α-hemolysin (α-HL) embedded in a lipid bilayer. Interactions between 18c6 and Na(+) produce characteristic pulse-like current amplitude signatures that allow the identification of individual AP sites in single molecules of homopolymeric or heteropolymeric DNA sequences. The bulky 18c6-cation complexes also dramatically slow the DNA motion to more easily recordable levels. Further, the behaviors of the AP-18c6 adduct are different with respect to the directionalities of DNA entering the protein channel, and they can be precisely manipulated by altering the cation (Li(+), Na(+) or K(+)) of the electrolyte. This method permits detection of multiple AP lesions per strand, which is unprecedented in other work. Additionally, insights into the thermodynamics and kinetics of 18c6-cation interactions at a single-molecule level are provided by the nanopore measurement.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Boiteux S, Guillet M. Abasic sites in DNA: Repair and biological consequences in Saccharomyces cerevisiae. DNA Repair. 2004;3:1–12. - PubMed
-
- Wilson DM, III, Barsky D. The major human abasic endonuclease: Formation, consequences and repair of abasic lesions in DNA. Mutat Res. 2001;485:283–307. - PubMed
-
- Kubo K, Ide H, Wallace SS, Kow YW. A novel, sensitive, and specific assay for abasic sites, the most commonly produced DNA lesion. Biochemistry. 1992;31:3703–3708. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

