Purified and synthetic Alzheimer's amyloid beta (Aβ) prions
- PMID: 22711819
- PMCID: PMC3390876
- DOI: 10.1073/pnas.1206555109
Purified and synthetic Alzheimer's amyloid beta (Aβ) prions
Abstract
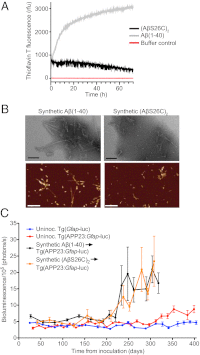
The aggregation and deposition of amyloid-β (Aβ) peptides are believed to be central events in the pathogenesis of Alzheimer's disease (AD). Inoculation of brain homogenates containing Aβ aggregates into susceptible transgenic mice accelerated Aβ deposition, suggesting that Aβ aggregates are capable of self-propagation and hence might be prions. Recently, we demonstrated that Aβ deposition can be monitored in live mice using bioluminescence imaging (BLI). Here, we use BLI to probe the ability of Aβ aggregates to self-propagate following inoculation into bigenic mice. We report compelling evidence that Aβ aggregates are prions by demonstrating widespread cerebral β-amyloidosis induced by inoculation of either purified Aβ aggregates derived from brain or aggregates composed of synthetic Aβ. Although synthetic Aβ aggregates were sufficient to induce Aβ deposition in vivo, they exhibited lower specific biological activity compared with brain-derived Aβ aggregates. Our results create an experimental paradigm that should lead to identification of self-propagating Aβ conformations, which could represent novel targets for interrupting the spread of Aβ deposition in AD patients.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
-
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. - PubMed
-
- Games D, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature. 1995;373:523–527. - PubMed
-
- Hsiao K, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

