Cutting edge: impaired MHC class I expression in mice deficient for Nlrc5/class I transactivator
- PMID: 22711889
- PMCID: PMC3578426
- DOI: 10.4049/jimmunol.1200064
Cutting edge: impaired MHC class I expression in mice deficient for Nlrc5/class I transactivator
Abstract
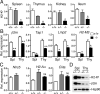
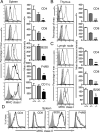
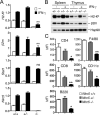
MHC class I and class II are crucial for the adaptive immune system. Although regulation of MHC class II expression by CIITA has long been recognized, the mechanism of MHC class I transactivation has been largely unknown until the recent discovery of NLRC5/class I transactivator. In this study, we show using Nlrc5-deficient mice that NLRC5 is required for both constitutive and inducible MHC class I expression. Loss of Nlrc5 resulted in severe reduction in the expression of MHC class I and related genes such as β(2)-microglobulin, Tap1, or Lmp2, but did not affect MHC class II levels. IFN-γ stimulation could not overcome the impaired MHC class I expression in Nlrc5-deficient cells. Upon infection with Listeria monocyogenes, Nlrc5-deficient mice displayed impaired CD8(+) T cell activation, accompanied with increased bacterial loads. These findings illustrate critical roles of NLRC5/class I transactivator in MHC class I gene regulation and host defense by CD8(+) T cell responses.
Figures

References
-
- Germain RN, Margulies DH. The biochemistry and cell biology of antigen processing and presentation. Annu. Rev. Immunol. 1993;11:403–450. - PubMed
-
- Steimle V, Otten LA, Zufferey M, Mach B. Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome) Cell. 1993;75:135–146. - PubMed
-
- Beresford GW, Boss JM. CIITA coordinates multiple histone acetylation modifications at the HLA-DRA promoter. Nat. Immunol. 2001;2:652–657. - PubMed
-
- Reith W, Mach B. The bare lymphocyte syndrome and the regulation of MHC expression. Annu. Rev. Immunol. 2001;19:331–373. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

