The complex actions of sumatriptan on rat dural afferents
- PMID: 22711897
- PMCID: PMC3583198
- DOI: 10.1177/0333102412451356
The complex actions of sumatriptan on rat dural afferents
Abstract
Aim: To test the hypothesis that the clinical efficacy of triptans reflects convergent modulation of ion channels also involved in inflammatory mediator (IM)-induced sensitization of dural afferents.
Methods: Acutely dissociated retrogradely labeled rat dural afferents were studied with whole cell and perforated patch techniques in the absence and presence of sumatriptan and/or IM (prostaglandin E2, bradykinin, and histamine).
Results: Sumatriptan dose-dependently suppressed voltage-gated Ca²⁺ currents. Acute (2 min) sumatriptan application increased dural afferent excitability and occluded further IM-induced sensitization. In contrast, pre-incubation (30 min) with sumatriptan had no influence on dural afferent excitability and partially prevented IM-induced sensitization of dural afferents. The sumatriptan-induced suppression of voltage-gated Ca²⁺ currents and acute sensitization and pre-incubation-induced block of IM-induced sensitization were blocked by the 5-HT(1D) antagonist BRL 15572. Pre-incubation with sumatriptan failed to suppress the IM-induced decrease in action potential threshold and overshoot (which results from modulation of voltage-gated Na⁺ currents) and activation of Cl⁻ current, and had no influence on the Cl⁻ reversal potential. However, pre-incubation with sumatriptan caused a dramatic hyperpolarizing shift in the voltage dependence of K⁺ current activation.
Discussion: These results indicate that although the actions of sumatriptan on dural afferents are complex, at least two distinct mechanisms underlie the antinociceptive actions of this compound. One of these mechanisms, the shift in the voltage dependence of K⁺ channel activation, may suggest a novel strategy for future development of anti-migraine agents.
Figures

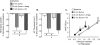
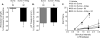


Similar articles
-
Electrophysiological properties of dural afferents in the absence and presence of inflammatory mediators.J Neurophysiol. 2009 Jun;101(6):3126-34. doi: 10.1152/jn.91339.2008. Epub 2009 Apr 1. J Neurophysiol. 2009. PMID: 19339455 Free PMC article.
-
Ionic mechanisms underlying inflammatory mediator-induced sensitization of dural afferents.J Neurosci. 2010 Jun 9;30(23):7878-88. doi: 10.1523/JNEUROSCI.6053-09.2010. J Neurosci. 2010. PMID: 20534836 Free PMC article.
-
Sumatriptan inhibits TRPV1 channels in trigeminal neurons.Headache. 2012 May;52(5):773-84. doi: 10.1111/j.1526-4610.2011.02053.x. Epub 2012 Jan 30. Headache. 2012. PMID: 22289052 Free PMC article.
-
Sex differences in the inflammatory mediator-induced sensitization of dural afferents.J Neurophysiol. 2011 Oct;106(4):1662-8. doi: 10.1152/jn.00196.2011. Epub 2011 Jul 13. J Neurophysiol. 2011. PMID: 21753025 Free PMC article.
-
Pharmacological synergy: the next frontier on therapeutic advancement for migraine.Headache. 2012 Apr;52(4):636-47. doi: 10.1111/j.1526-4610.2011.02058.x. Epub 2012 Jan 6. Headache. 2012. PMID: 22221151 Review.
Cited by
-
Animal models of migraine and experimental techniques used to examine trigeminal sensory processing.J Headache Pain. 2019 Aug 29;20(1):91. doi: 10.1186/s10194-019-1043-7. J Headache Pain. 2019. PMID: 31464579 Free PMC article. Review.
-
Release of CGRP from mouse brainstem slices indicates central inhibitory effect of triptans and kynurenate.J Headache Pain. 2014 Feb 8;15(1):7. doi: 10.1186/1129-2377-15-7. J Headache Pain. 2014. PMID: 24506953 Free PMC article.
-
Optogenetic Spreading Depression Elicits Trigeminal Pain and Anxiety Behavior.Ann Neurol. 2021 Jan;89(1):99-110. doi: 10.1002/ana.25926. Epub 2020 Oct 27. Ann Neurol. 2021. PMID: 33016466 Free PMC article.
-
A PTEN-Regulated Checkpoint Controls Surface Delivery of δ Opioid Receptors.J Neurosci. 2017 Apr 5;37(14):3741-3752. doi: 10.1523/JNEUROSCI.2923-16.2017. Epub 2017 Mar 6. J Neurosci. 2017. PMID: 28264976 Free PMC article.
-
Serotonin, 5HT1 agonists, and migraine: new data, but old questions still not answered.Curr Opin Support Palliat Care. 2014 Jun;8(2):137-42. doi: 10.1097/SPC.0000000000000044. Curr Opin Support Palliat Care. 2014. PMID: 24670810 Free PMC article. Review.
References
-
- Lipton RB, Stewart WF, Scher AI. Epidemiology and economic impact of migraine. Curr Med Res Opin. 2001;17(Suppl 1):s4–12. - PubMed
-
- Galletti F, Cupini LM, Corbelli I, Calabresi P, Sarchielli P. Pathophysiological basis of migraine prophylaxis. Prog Neurobiol. 2009;89:176–92. - PubMed
-
- Tfelt-Hansen P, De Vries P, Saxena PR. Triptans in migraine: a comparative review of pharmacology, pharmacokinetics and efficacy. Drugs. 2000;60:1259–87. - PubMed
-
- Sarchielli P, Alberti A, Codini M, Floridi A, Gallai V. Nitric oxide metabolites, prostaglandins and trigeminal vasoactive peptides in internal jugular vein blood during spontaneous migraine attacks. Cephalalgia. 2000;20:907–18. - PubMed
-
- Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature. 1996;384:560–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

