Human papillomavirus vaccination in Tanzanian schoolgirls: cluster-randomized trial comparing 2 vaccine-delivery strategies
- PMID: 22711908
- PMCID: PMC3414230
- DOI: 10.1093/infdis/jis407
Human papillomavirus vaccination in Tanzanian schoolgirls: cluster-randomized trial comparing 2 vaccine-delivery strategies
Abstract
Background: We compared vaccine coverage achieved by 2 different delivery strategies for the quadrivalent human papillomavirus (HPV) vaccine in Tanzanian schoolgirls.
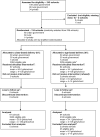
Methods: In a cluster-randomized trial of HPV vaccination conducted in Tanzania, 134 primary schools were randomly assigned to class-based (girls enrolled in primary school grade [class] 6) or age-based (girls born in 1998; 67 schools per arm) vaccine delivery. The primary outcome was coverage by dose.
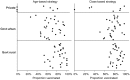
Results: There were 3352 and 2180 eligible girls in schools randomized to class-based and age-based delivery, respectively. HPV vaccine coverage was 84.7% for dose 1, 81.4% for dose 2, and 76.1% for dose 3. For each dose, coverage was higher in class-based schools than in age-based schools (dose 1: 86.4% vs 82.0% [P = .30]; dose 2: 83.8% vs 77.8% [P = .05]; and dose 3: 78.7% vs 72.1% [P = .04]). Vaccine-related adverse events were rare. Reasons for not vaccinating included absenteeism (6.3%) and parent refusal (6.7%). School absenteeism rates prior to vaccination ranged from 8.1% to 23.5%.
Conclusions: HPV vaccine can be delivered with high coverage in schools in sub-Saharan Africa. Compared with age-based vaccination, class-based vaccination located more eligible pupils and achieved higher coverage. HPV vaccination did not increase absenteeism rates in selected schools. Innovative strategies will be needed to reach out-of-school girls.
Clinical trials registration: NCT01173900.
Figures
References
-
- Koutsky L. Epidemiology of genital human papillomavirus infection. Am J Med. 1997;102:3–8. - PubMed
-
- Walbloomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. - PubMed
-
- Ferlay JSH, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. - PubMed
-
- GAVI Alliance. GAVI investment strategy. 2008 Human papillomavirus analysis. http://www.gavialliance.org/search/?SearchFor=0&SearchText=Landscape+ana... . Accessed 3 February 2012.

