Defective mitochondrial morphology and bioenergetic function in mice lacking the transcription factor Yin Yang 1 in skeletal muscle
- PMID: 22711985
- PMCID: PMC3434543
- DOI: 10.1128/MCB.00337-12
Defective mitochondrial morphology and bioenergetic function in mice lacking the transcription factor Yin Yang 1 in skeletal muscle
Abstract
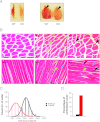
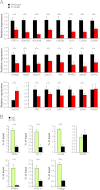
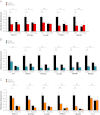
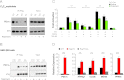
The formation, distribution, and maintenance of functional mitochondria are achieved through dynamic processes that depend strictly on the transcription of nuclear genes encoding mitochondrial proteins. A large number of these mitochondrial genes contain binding sites for the transcription factor Yin Yang 1 (YY1) in their proximal promoters, but the physiological relevance is unknown. We report here that skeletal-muscle-specific YY1 knockout (YY1mKO) mice have severely defective mitochondrial morphology and oxidative function associated with exercise intolerance, signs of mitochondrial myopathy, and short stature. Gene set enrichment analysis (GSEA) revealed that the top pathways downregulated in YY1mKO mice were assigned to key metabolic and regulatory mitochondrial genes. This analysis was consistent with a profound decrease in the level of mitochondrial proteins and oxidative phosphorylation (OXPHOS) bioenergetic function in these mice. In contrast to the finding for wild-type mice, inactivation of the mammalian target of rapamycin (mTOR) did not suppress mitochondrial genes in YY1mKO mice. Mechanistically, mTOR-dependent phosphorylation of YY1 resulted in a strong interaction between YY1 and the transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator 1α (PGC1α), a major regulator of mitochondrial function. These results underscore the important role of YY1 in the maintenance of mitochondrial function and explain how its inactivation might contribute to exercise intolerance and mitochondrial myopathies.
Figures





References
-
- Austen M, Luscher B, Luscher-Firzlaff JM. 1997. Characterization of the transcriptional regulator YY1. The bipartite transactivation domain is independent of interaction with the TATA box-binding protein, transcription factor IIB, TAFII55, or cAMP-responsive element-binding protein (CPB)-binding protein. J. Biol. Chem. 272:1709–1717 - PubMed
-
- Bentzinger CF, et al. 2008. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab. 8:411–424 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
