Anatomy of Graft-induced Dyskinesias: Circuit Remodeling in the Parkinsonian Striatum
- PMID: 22712056
- PMCID: PMC3375918
- DOI: 10.1016/j.baga.2012.01.002
Anatomy of Graft-induced Dyskinesias: Circuit Remodeling in the Parkinsonian Striatum
Abstract
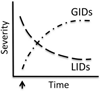
The goal of researchers and clinicians interested in re-instituting cell based therapies for PD is to develop an effective and safe surgical approach to replace dopamine (DA) in individuals suffering from Parkinson's disease (PD). Worldwide clinical trials involving transplantation of embryonic DA neurons into individuals with PD have been discontinued because of the often devastating post-surgical side-effect known as graft-induced dyskinesia (GID). There have been many review articles published in recent years on this subject. There has been a tendency to promote single factors in the cause of GID. In this review, we contrast the pros and cons of multiple factors that have been suggested from clinical and/or preclinical observations, as well as novel factors not yet studied that may be involved with GID. It is our intention to provide a platform that might be instrumental in examining how individual factors that correlate with GID and/or striatal pathology might interact to give rise to dysfunctional circuit remodeling and aberrant motor output.
Figures


References
-
- Dunn EH. Primary and secondary findings in a series of attempts to transplant cerebral cortex in the albino rat. J Comp Neurol. 1917;27:565–582.
-
- Lindvall O, Brundin P, Widner H, Rehncrona S, Gustavii B, Frackowiak R, et al. Grafts of fetal dopamine neurons survive and improve motor function in Parkinson’s disease. Science. 1990;247:574–577. - PubMed
-
- Freed CR, Breeze RE, Rosenberg NL, Schneck SA, Kriek E, Qi JX, et al. Survival of implanted fetal dopamine cells and neurologic improvement 12 to 46 months after transplantation for Parkinson’s disease. N Engl J Med. 1992;327:1549–1555. - PubMed
-
- Lindvall O, Sawle G, Widner H, Rothwell JC, Björklund A, Brooks D, et al. Evidence for long-term survival and function of dopaminergic grafts in progressive Parkinson’s disease. Ann Neurol. 1994;35:172–180. - PubMed
-
- Peschanski M, Defer G, N’Guyen JP, Ricolfi F, Monfort JC, Remy P, et al. Bilateral motor improvement and alteration of L-dopa effect in two patients with Parkinson’s disease following intrastriatal transplantation of foetal ventral mesencephalon. Brain. 1994;117:487–499. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
