Intramolecular hydrogen transfer reactions of thiyl radicals from glutathione: formation of carbon-centered radical at Glu, Cys, and Gly
- PMID: 22712461
- PMCID: PMC3736831
- DOI: 10.1021/tx3000494
Intramolecular hydrogen transfer reactions of thiyl radicals from glutathione: formation of carbon-centered radical at Glu, Cys, and Gly
Abstract
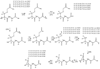
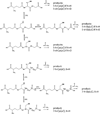
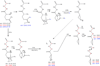
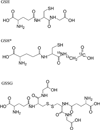
Glutathione thiyl radicals (GS(•)) were generated in H(2)O and D(2)O by either exposure of GSH to AAPH, photoirradiation of GSH in the presence of acetone, or photoirradiation of GSSG. Detailed interpretation of the fragmentation pathways of deuterated GSH and GSH derivatives during mass spectrometry analysis allowed us to demonstrate that reversible intramolecular H-atom transfer reactions between GS(•) and C-H bonds at Cys[(α)C], Cys[(β)C], and Gly[(α)C] are possible.
Figures












Similar articles
-
Reversible hydrogen transfer between cysteine thiyl radical and glycine and alanine in model peptides: covalent H/D exchange, radical-radical reactions, and L- to D-Ala conversion.J Phys Chem B. 2010 May 20;114(19):6751-62. doi: 10.1021/jp101508b. J Phys Chem B. 2010. PMID: 20415493
-
Hydrogen exchange equilibria in thiols.Chem Res Toxicol. 2012 Sep 17;25(9):1862-7. doi: 10.1021/tx300045f. Epub 2012 Jul 3. Chem Res Toxicol. 2012. PMID: 22712484
-
Reversible hydrogen transfer reactions of cysteine thiyl radicals in peptides: the conversion of cysteine into dehydroalanine and alanine, and of alanine into dehydroalanine.J Phys Chem B. 2011 Oct 27;115(42):12287-305. doi: 10.1021/jp2070453. Epub 2011 Sep 30. J Phys Chem B. 2011. PMID: 21895001 Free PMC article.
-
Cysteine residues as catalysts for covalent peptide and protein modification: a role for thiyl radicals?Biochem Soc Trans. 2011 Oct;39(5):1254-9. doi: 10.1042/BST0391254. Biochem Soc Trans. 2011. PMID: 21936798 Free PMC article. Review.
-
Protein thiyl radical reactions and product formation: a kinetic simulation.Free Radic Biol Med. 2015 Mar;80:158-63. doi: 10.1016/j.freeradbiomed.2014.12.006. Epub 2014 Dec 12. Free Radic Biol Med. 2015. PMID: 25499854 Free PMC article. Review.
Cited by
-
Probing the Role of Cysteine Thiyl Radicals in Biology: Eminently Dangerous, Difficult to Scavenge.Antioxidants (Basel). 2022 Apr 29;11(5):885. doi: 10.3390/antiox11050885. Antioxidants (Basel). 2022. PMID: 35624747 Free PMC article. Review.
-
Oxidation of ethidium-based probes by biological radicals: mechanism, kinetics and implications for the detection of superoxide.Sci Rep. 2020 Oct 29;10(1):18626. doi: 10.1038/s41598-020-75373-2. Sci Rep. 2020. PMID: 33122809 Free PMC article.
-
Thiyl radicals and induction of protein degradation.Free Radic Res. 2016;50(2):143-9. doi: 10.3109/10715762.2015.1077385. Epub 2015 Aug 28. Free Radic Res. 2016. PMID: 26212409 Free PMC article. Review.
-
Light-Induced Covalent Buffer Adducts to Histidine in a Model Protein.Pharm Res. 2018 Feb 20;35(3):67. doi: 10.1007/s11095-017-2339-4. Pharm Res. 2018. PMID: 29464419
-
Thiyl Radical Reactions in the Chemical Degradation of Pharmaceutical Proteins.Molecules. 2019 Nov 28;24(23):4357. doi: 10.3390/molecules24234357. Molecules. 2019. PMID: 31795282 Free PMC article. Review.
References
-
- Oakley A. Glutathione transferases: a structural perspective. Drug Metab Rev. 2011;43:138–151. - PubMed
-
- Gilbert HF. Thiol/Disulfide Exchange Equilibria and Disulfide Bond Stability. Methods Enzymol. 1995;251:8–28. - PubMed
-
- Ross D, Albano E, Nilsson U, Moldeus P. Thiyl Radicals - Formation during Peroxidase-Catalyzed Metabolism of Acetaminophen in the Presence of Thiols. Biochem Biophys Res Commun. 1984;125:109–115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

