Intramolecular hydrogen transfer reactions of thiyl radicals from glutathione: formation of carbon-centered radical at Glu, Cys, and Gly
- PMID: 22712461
- PMCID: PMC3736831
- DOI: 10.1021/tx3000494
Intramolecular hydrogen transfer reactions of thiyl radicals from glutathione: formation of carbon-centered radical at Glu, Cys, and Gly
Abstract
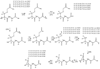
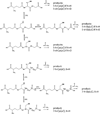
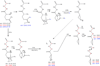
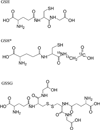
Glutathione thiyl radicals (GS(•)) were generated in H(2)O and D(2)O by either exposure of GSH to AAPH, photoirradiation of GSH in the presence of acetone, or photoirradiation of GSSG. Detailed interpretation of the fragmentation pathways of deuterated GSH and GSH derivatives during mass spectrometry analysis allowed us to demonstrate that reversible intramolecular H-atom transfer reactions between GS(•) and C-H bonds at Cys[(α)C], Cys[(β)C], and Gly[(α)C] are possible.
Figures












References
-
- Oakley A. Glutathione transferases: a structural perspective. Drug Metab Rev. 2011;43:138–151. - PubMed
-
- Gilbert HF. Thiol/Disulfide Exchange Equilibria and Disulfide Bond Stability. Methods Enzymol. 1995;251:8–28. - PubMed
-
- Ross D, Albano E, Nilsson U, Moldeus P. Thiyl Radicals - Formation during Peroxidase-Catalyzed Metabolism of Acetaminophen in the Presence of Thiols. Biochem Biophys Res Commun. 1984;125:109–115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

