Synthetic oligosaccharides as tools to demonstrate cross-reactivity between polysaccharide antigens
- PMID: 22713129
- PMCID: PMC3746347
- DOI: 10.1021/jo300299p
Synthetic oligosaccharides as tools to demonstrate cross-reactivity between polysaccharide antigens
Abstract
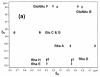
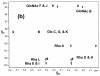
Escherichia coli O148 is a nonencapsulated enterotoxigenic (ETEC) Gram negative bacterium that can cause diarrhea, hemorrhagic colitis, and hemolytic uremic syndrome in humans. The surface-exposed O-specific polysaccharide (O-SP) of the lipopolysaccharide of this bacterium is considered both a virulence factor and a protective antigen. It is built up of the linear tetrasaccharide repeating unit [3)-α-L-Rhap-(1→2)-α-D-Glcp-(1→3)-α-D-GlcNAcp-(1→3)-α-L-Rhap-(1→] differing from that of the O-SP of Shigella dysenteriae type 1 (SD) only in that the latter contains a D-Galp residue in place of the glucose moiety of the former. The close similarity of the O-SPs of these bacteria indicated a possible cross-reactivity. To answer this question we synthesized several oligosaccharide fragments of E. coli O148 O-SP, up to a dodecasaccharide, as well as their bovine serum albumin or recombinant diphtheria toxin conjugates. Immunization of mice with these conjugates induced anti-O-SP-specific serum IgG antibody responses. The antisera reacted equally well with the LPSs of both bacteria, indicating cross-reactivity between the SD and E. coli O148 O-SPs that was further supported by Western-blot and dot-blot analyses, as well as by inhibition of binding between the antisera and the O-SPs of both bacteria.
Figures













References
-
- Rowe B, Taylor J, Bettelhe KA. Lancet. 1970;1:1. - PubMed
-
- Espie E, Grimont F, Montet MP, Carle I, Bavai C, de Valk H. Clin. Microbiol. Invest. 2006;12:992–998. - PubMed
-
- Viazis S, Diez-Gonzalez F. Enterohemorrhagic Escherichia Coli: the Twentieth Century'S Emerging Foodborne Pathogen: A Review. In: Sparks DL, editor. Advances in Agronomy. 2011. pp. 1–50.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

