Mitochondrial fusion by pharmacological manipulation impedes somatic cell reprogramming to pluripotency: new insight into the role of mitophagy in cell stemness
- PMID: 22713507
- PMCID: PMC3409676
- DOI: 10.18632/aging.100465
Mitochondrial fusion by pharmacological manipulation impedes somatic cell reprogramming to pluripotency: new insight into the role of mitophagy in cell stemness
Abstract
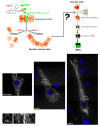
Recent studies have suggested a pivotal role for autophagy in stem cell maintenance and differentiation. Reprogramming of somatic cells to induced pluripotent stem cells (iPSCs) has been also suggested to bio-energetically take advantage of mitochondrial autophagy (mitophagy). We have preliminary addressed how mitophagy might play a role in the regulation of induced pluripotency using mdivi-1 (for mitochondrial division inhibitor), a highly efficacious small molecule that selectively inhibits the self-assembly of DRP1, a member of the dynamin family of large GTPases that mediates mitochondrial fission. At mdivi-1 concentrations that rapidly induced the formation of mitochondrial net-like or collapsed perinuclear mitochondrial structures, we observed that the reprogramming efficiency of mouse embryonic fibroblasts transduced with the Yamanaka three-factor cocktail (OCT4, KLF4, and SOX2) is drastically reduced by more than 95%. Treatment of MEFs with mdivi-1 at the early stages of reprogramming before the appearance of iPSC colonies was sufficient to completely inhibit somatic cell reprogramming. Therefore, the observed effects on reprogramming efficiencies were due likely to the inhibition of the process of reprogramming itself and not to an impairment of iPSC colony survival or growth. Moreover, the typical morphology of established iPSC colonies with positive alkaline phosphatase staining was negatively affected by mdivi-1 exposure. In the presence of mdivi-1, the colony morphology of the iPSCs was lost, and they somewhat resembled fibroblasts. The alkaline phosphatase staining was also significantly reduced, a finding that is indicative of differentiation. Our current findings provide new insight into how mitochondrial division is integrated into the reprogramming factors-driven transcriptional network that specifies the unique pluripotency of stem cells.
Conflict of interest statement
The authors of this manuscript have no conflict of interest to declare.
Figures


Similar articles
-
Mitochondrial division inhibitor (mdivi-1) decreases oxidative metabolism in cancer.Br J Cancer. 2020 Apr;122(9):1288-1297. doi: 10.1038/s41416-020-0778-x. Epub 2020 Mar 9. Br J Cancer. 2020. PMID: 32147668 Free PMC article.
-
Silibinin-induced mitochondria fission leads to mitophagy, which attenuates silibinin-induced apoptosis in MCF-7 and MDA-MB-231 cells.Arch Biochem Biophys. 2020 May 30;685:108284. doi: 10.1016/j.abb.2020.108284. Epub 2020 Jan 31. Arch Biochem Biophys. 2020. PMID: 32014401
-
Mouse Embryonic Fibroblasts Reprogramming to Induced Pluripotent Stem Cells by T3.Methods Mol Biol. 2025;2876:117-130. doi: 10.1007/978-1-0716-4252-8_8. Methods Mol Biol. 2025. PMID: 39579312
-
mTOR-regulated senescence and autophagy during reprogramming of somatic cells to pluripotency: a roadmap from energy metabolism to stem cell renewal and aging.Cell Cycle. 2011 Nov 1;10(21):3658-77. doi: 10.4161/cc.10.21.18128. Epub 2011 Nov 1. Cell Cycle. 2011. PMID: 22052357 Review.
-
Understanding the roadmaps to induced pluripotency.Cell Death Dis. 2014 May 15;5(5):e1232. doi: 10.1038/cddis.2014.205. Cell Death Dis. 2014. PMID: 24832604 Free PMC article. Review.
Cited by
-
The Warburg effect version 2.0: metabolic reprogramming of cancer stem cells.Cell Cycle. 2013 Apr 15;12(8):1166-79. doi: 10.4161/cc.24479. Epub 2013 Apr 2. Cell Cycle. 2013. PMID: 23549172 Free PMC article.
-
Connecting Mitochondria, Metabolism, and Stem Cell Fate.Stem Cells Dev. 2015 Sep 1;24(17):1957-71. doi: 10.1089/scd.2015.0117. Epub 2015 Jul 2. Stem Cells Dev. 2015. PMID: 26134242 Free PMC article. Review.
-
Mitostemness.Cell Cycle. 2018;17(8):918-926. doi: 10.1080/15384101.2018.1467679. Epub 2018 Jul 2. Cell Cycle. 2018. PMID: 29886796 Free PMC article. Review.
-
Mitochondrial division inhibitor 1 (mdivi-1) increases oxidative capacity and contractile stress generated by engineered skeletal muscle.FASEB J. 2020 Sep;34(9):11562-11576. doi: 10.1096/fj.201901039RR. Epub 2020 Jul 11. FASEB J. 2020. PMID: 32652761 Free PMC article.
-
Metabolome and metaboproteome remodeling in nuclear reprogramming.Cell Cycle. 2013 Aug 1;12(15):2355-65. doi: 10.4161/cc.25509. Epub 2013 Jul 8. Cell Cycle. 2013. PMID: 23839047 Free PMC article.
References
-
- Di Gioacchino M, Petrarca C, Perrone A, Martino S, Esposito DL, Lotti LV, Mariani-Costantini R. Autophagy in hematopoietic stem/progenitor cells exposed to heavy metals: Biological implications and toxicological relevance. Autophagy. 2008;4:537–539. - PubMed
-
- Liu F, Guan JL. FIP200, an essential component of mammalian autophagy is indispensible for fetal hematopoiesis. Autophagy. 2011;7:229–230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

