The unfolded protein response is activated in connexin 50 mutant mouse lenses
- PMID: 22713599
- PMCID: PMC3461258
- DOI: 10.1016/j.exer.2012.06.004
The unfolded protein response is activated in connexin 50 mutant mouse lenses
Abstract
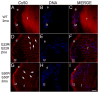
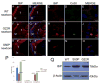
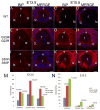
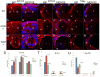
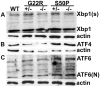
The unfolded protein response is a set of cell signaling pathways recently recognized to be activated in the lens during both normal development and endoplasmic reticulum stress induced by either unfolded proteins or oxidative damage. While mutations in the gene for connexin 50 are known to cause autosomal dominant cataracts, it has not been previously reported whether mutant connexins can activate the unfolded protein response in the lens. Mice homozygous for the S50P or G22R mutation of connexin 50 have reduced amounts of connexin 50 protein at the cell membrane, with some intracellular staining consistent with retention in the endoplasmic reticulum. Connexin 50 mutants have elevated levels of BiP expression in both lens epithelial and fiber cells from E15.5 with the most robust elevation detected in newborns. While this elevation decreases in magnitude postnatally, BiP expression is still abnormally high in adults, particularly in the perinuclear endoplasmic reticulum of cell nuclei that are inappropriately retained in adult homozygous mutant lenses. Xbp1 splicing was elevated in lenses from both connexin mutants studied, while Atf4 and Atf6 levels were not majorly affected. Overall, these data suggest that UPR may be a contributing factor to the phenotype of connexin 50 mutant lenses even though the relatively modest extent of the response suggests that it is unlikely to be a major driver of the pathology.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures



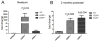
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

