Mixed lineage leukaemia-4 regulates cell-cycle progression and cell viability and its depletion suppresses growth of xenografted tumour in vivo
- PMID: 22713656
- PMCID: PMC3394987
- DOI: 10.1038/bjc.2012.263
Mixed lineage leukaemia-4 regulates cell-cycle progression and cell viability and its depletion suppresses growth of xenografted tumour in vivo
Abstract
Background: Mixed lineage leukaemia-4 (MLL4) is one of the MLL family of histone H3 lysine-4 (H3K4)-specific methyl transferases that have critical roles in gene expression and epigenetics in human. Though MLLs are well recognised as crucial players in histone methylation and gene regulation; little is known about the biochemical functions of MLL4 and its roles in cancer.
Methods: Herein, we have investigated the roles of MLL4 in cell viability, cell-cycle progression and explored its potential roles in tumour growth using antisense-mediated knockdown experiments, flow-cytometry analysis, chromatin immunoprecipitation, immunofluorescence staining and animal models.
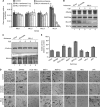
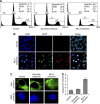
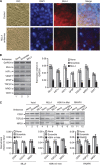
Results: Our studies demonstrated that knockdown of MLL4 severely affects cell-cycle progression and induces apoptotic cell death in cultured tumour cells. Knockdown of MLL4 induced nuclear condensation, fragmentation, cytochrome-c release from mitochondria to cytosol and activated caspase-3/7 indicating apoptotic cell death. The MLL4 regulates expression of various critical cell-cycle regulatory genes such as cyclin D, cyclin E, p27, HOXA5 and HOXB7 via histone H3K4 trimethylation and recruitment of RNA polymerase II. Interestingly, application of MLL4 antisense suppressed tumour growth in vivo in colon cancer xenograft implanted in nude mouse. The MLL4 antisense specifically knocked down MLL4 in tumour tissue and also downregulated the expression of various growth and angiogenic factors resulting in tumour suppression.
Conclusion: Our results demonstrated that MLL4 is a crucial player in cell viability, cell-cycle progression and is critical for tumour growth in vivo.
Figures


References
-
- Ajiro K, Allis CD (2002) Histone code hypothesis. Tanpakushitsu Kakusan Koso 47: 753–760 - PubMed
-
- Ansari KI, Grant JD, Woldemariam GA, Kasiri S, Mandal SS (2009) Iron(III)-salen complexes with less DNA cleavage activity exhibit more efficient apoptosis in MCF7 cells. Org Biomol Chem 7: 926–932 - PubMed
-
- Ansari KI, Hussain I, Kasiri S, Mandal SS (2012) HOXC10 is overexpressed in breast cancer and transcriptionally regulated by estrogen via involvement of histone methylases MLL3 and MLL4. J Mol Endocrinol 48: 61–75 - PubMed
-
- Ansari KI, Mandal SS (2010) Mixed lineage leukemia: roles in gene expression, hormone signaling and mRNA processing. FEBS J 277: 1790–1804 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

