Harmonization of the intracellular cytokine staining assay
- PMID: 22714399
- PMCID: PMC3378841
- DOI: 10.1007/s00262-012-1282-9
Harmonization of the intracellular cytokine staining assay
Abstract
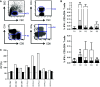
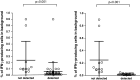
Active immunotherapy for cancer is an accepted treatment modality aiming to reinforce the T-cell response to cancer. T-cell reactivity is measured by various assays and used to guide the clinical development of immunotherapeutics. However, data obtained across different institutions may vary substantially making comparative conclusions difficult. The Cancer Immunotherapy Immunoguiding Program organizes proficiency panels to identify key parameters influencing the outcome of commonly used T-cell assays followed by harmonization. Our successes with IFNγ-ELISPOT and peptide HLA multimer analysis have led to the current study on intracellular cytokine staining (ICS). We report the results of three successive panels evaluating this assay. At the beginning, 3 out of 9 participants (33 %) were able to detect >6 out of 8 known virus-specific T-cell responses in peripheral blood of healthy individuals. This increased to 50 % of the laboratories in the second phase. The reported percentages of cytokine-producing T cells by the different laboratories were highly variable with coefficients of variation well over 60 %. Variability could partially be explained by protocol-related differences in background cytokine production leading to sub-optimal signal-to-noise ratios. The large number of protocol variables prohibited identification of prime guidelines to harmonize the assays. In addition, the gating strategy used to identify reactive T cells had a major impact on assay outcome. Subsequent harmonization of the gating strategy considerably reduced the variability within the group of participants. In conclusion, we propose that first basic guidelines should be applied for gating in ICS experiments before harmonizing assay protocol variables.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky Am Mlecnik B, Lagorce-Page C, Tosolini M, Camus M, Berger A, Wind Ph, Zinzindohoue F, Bruneval P, Cugnenc H, Trajanoski Z, Fridman WH, Page F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. - DOI - PubMed
-
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF, Ahmed T, Amin A, Arseneau J, Barth N, Bernstein G, Bracken B, Burch P, Caggiano V, Chin J, Chodak G, Chu F, Corman J, Curti B, Dawson N, Deeken JF, Dubernet T, Fishman M, Flanigan R, Gailani F, Garbo L, Gardner T, Gelmann E, George D, Godfrey T, Gomella L, Guerra M, Hall S, Hanson J, Israeli R, Jancis E, Jewett MAS, Kassabian V, Katz J, Klotz L, Koeneman K, Koh H, Kratzke R, Lance R, Lech J, Leichman L, Lemon R, Liang J, Libertino J, Lilly M, Malik I, Martin SE, McCaffrey J, McLeod D, McNeel D, Miles B, Murdock M, Nabhan C, Nemunaitis J, Notter D, Pantuck A, Perrotte P, Pessis D, Petrylak D, Polikoff J, Pommerville P, Ramanathan S, Rarick M, Richards J, Rifkin R, Rohatgi N, Rosenbluth R, Santucci R, Sayegh A, Seigne J, Shapira I, Shedhadeh N, Shepherd D, Sridhar S, Stephenson R, Teigland C, Thaker N, Vacirca J, Villa L, Vogelzang N, Wertheim M, Wolff JH, Wurzel R, Yang C, Young J. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. - DOI - PubMed
-
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJM, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with Ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. - DOI - PMC - PubMed
-
- Welters MJ, Kenter GG, de Vos van Steenwijk PJ, Löwik MJ, Berends-van der Meer DM, Essahsah F, Stynenbosch LF, Vloon AP, Ramwadhdoebe TH, Piersma SJ, van der Hulst JM, Valentijn AR, Fathers LM, Drijfhout JW, Franken KL, Oostendorp J, Fleuren GJ, Melief CJ, van der Burg SH. Success or failure of vaccination for HPV16-positive vulvar lesions correlates with kinetics and phenotype of induced T-cell responses. Proc Nat Acad Sci USA. 2010;107:11895–11899. doi: 10.1073/pnas.1006500107. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

