Design and rationale of the LAPLACE-TIMI 57 trial: a phase II, double-blind, placebo-controlled study of the efficacy and tolerability of a monoclonal antibody inhibitor of PCSK9 in subjects with hypercholesterolemia on background statin therapy
- PMID: 22714699
- PMCID: PMC4347804
- DOI: 10.1002/clc.22014
Design and rationale of the LAPLACE-TIMI 57 trial: a phase II, double-blind, placebo-controlled study of the efficacy and tolerability of a monoclonal antibody inhibitor of PCSK9 in subjects with hypercholesterolemia on background statin therapy
Abstract
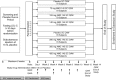
Lowering low-density lipoprotein cholesterol (LDL-C) is a cornerstone for the prevention of atherosclerotic heart disease, improving clinical outcomes and reducing vascular mortality in patients with hypercholesterolemia. The clinical benefits of LDL-C reduction appear to extend even to patients starting with LDL-C as low as 60-80 mg/dL prior to initiating therapy. Statins are the first-line agents for treating hypercholesterolemia and are effective in reducing LDL-C, but many patients are unable to achieve their optimal lipid targets despite intensive statin therapy. Therefore, there has been a strong impetus for the development of novel pharmacologic agents designed to lower LDL-C further in patients already on statin therapy. Genetic mutations resulting in altered cholesterol homeostasis provide valuable information regarding novel approaches for treating hypercholesterolemia. To that end, mutations in proprotein convertase subtilisin/kexin type 9 (PCSK9) were linked to altered levels of LDL-C, illustrating this protein's role in lipid metabolism. PCSK9 promotes degradation of the LDL receptor, preventing its transport back to the cell surface and thereby increasing circulating LDL-C. Conversely, inhibition of PCSK9 can profoundly decrease circulating LDL-C, and thus is an attractive new target for LDL-C-lowering therapy. AMG 145 is a fully human monoclonal immunoglobulin G2 antibody that binds specifically to human PCSK9 and inhibits its interaction with the low-density lipoprotein receptor. In this manuscript, we describe the rationale and design of LDL-C Assessment with PCSK9 Monoclonal Antibody Inhibition Combined With Statin Therapy-Thrombolysis In Myocardial Infarction 57 (LAPLACE-TIMI 57; NCT01380730), a 12-week, randomized, double-blind, dose-ranging, placebo-controlled study designed to assess the safety and efficacy of AMG 145 when added to statin therapy in patients with hypercholesterolemia.
© 2012 Wiley Periodicals, Inc.
Figures
References
-
- Lloyd‐Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:948–954. - PubMed
-
- Reiner Z, Catapano AL, De Backer G, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32:1769–1818. - PubMed
-
- Baigent C, Keech A, Kearney PM, et al; Cholesterol Treatment Triallists' Collaborators. Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90 056 participants in 14 randomised trials of statins [published corrections appear in Lancet. 2005;366:1358 and 2008;371:2084]. Lancet. 2005;366:1267–1278. - PubMed
-
- Kearney PM, Blackwell L, Collins R, et al; Cholesterol Treatment Triallists' Collaborators. Efficacy of cholesterol‐lowering therapy in 18 686 people with diabetes in 14 randomised trials of statins: a meta‐analysis. Lancet. 2008;371:117–125. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

