Developmental expression of the neuron-specific N-acetylglucosaminyltransferase Vb (GnT-Vb/IX) and identification of its in vivo glycan products in comparison with those of its paralog, GnT-V
- PMID: 22715095
- PMCID: PMC3436567
- DOI: 10.1074/jbc.M112.367565
Developmental expression of the neuron-specific N-acetylglucosaminyltransferase Vb (GnT-Vb/IX) and identification of its in vivo glycan products in comparison with those of its paralog, GnT-V
Abstract
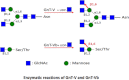
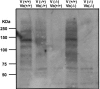
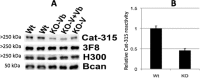
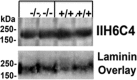
The severe phenotypic effects of altered glycosylation in the congenital muscular dystrophies, including Walker-Warburg syndrome, muscle-eye-brain disease, Fukuyama congenital muscular dystrophy, and congenital muscular dystrophy 1D, are caused by mutations resulting in altered glycans linked to proteins through O-linked mannose. A glycosyltransferase that branches O-Man, N-acetylglucosaminyltransferase Vb (GnT-Vb), is highly expressed in neural tissues. To understand the expression and function of GnT-Vb, we studied its expression during neuromorphogenesis and generated GnT-Vb null mice. A paralog of GnT-Vb, N-acetylglucosaminyltransferase (GnT-V), is expressed in many tissues and brain, synthesizing N-linked, β1,6-branched glycans, but its ability to synthesize O-mannosyl-branched glycans is unknown; conversely, although GnT-Vb can synthesize N-linked glycans in vitro, its contribution to their synthesis in vivo is unknown. Our results showed that deleting both GnT-V and GnT-Vb results in the total loss of both N-linked and O-Man-linked β1,6-branched glycans. GnT-V null brains lacked N-linked, β1,6-glycans but had normal levels of O-Man β1,6-branched structures, showing that GnT-Vb could not compensate for the loss of GnT-V. By contrast, GnT-Vb null brains contained normal levels of N-linked β1,6-glycans but low levels of some O-Man β1,6-branched glycans. Therefore, GnT-V could partially compensate for GnT-Vb activity in vivo. We found no apparent change in α-dystroglycan binding of glycan-specific antibody IIH6C4 or binding to laminin in GnT-Vb null mice. These results demonstrate that GnT-V is involved in synthesizing branched O-mannosyl glycans in brain, but the function of these branched O-mannosyl structures is unresolved using mice that lack these glycosyltransferases.
Figures


References
-
- Dobyns W. B., Kirkpatrick J. B., Hittner H. M., Roberts R. M., Kretzer F. L. (1985) Syndromes with lissencephaly. II. Walker-Warburg and cerebro-oculo-muscular syndromes and a new syndrome with type II lissencephaly. Am. J. Med. Genet. 22, 157–195 - PubMed
-
- Golden J. A. (2001) Cell migration and cerebral cortical development. Neuropathol. Appl. Neurobiol. 27, 22–28 - PubMed
-
- Haltia M., Leivo I., Somer H., Pihko H., Paetau A., Kivelä T., Tarkkanen A., Tom F., Engvall E., Santavuori P. (1997) Muscle-eye-brain disease. A neuropathological study. Ann. Neurol. 41, 173–180 - PubMed
-
- Lian G., Sheen V. (2006) Cerebral developmental disorders. Curr. Opin. Pediatr. 18, 614–620 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

