Pulmonary diffuse alveolar septal amyloidosis--diagnosed by transbronchial lung biopsy
- PMID: 2271513
- PMCID: PMC4534991
- DOI: 10.3904/kjim.1990.5.1.63
Pulmonary diffuse alveolar septal amyloidosis--diagnosed by transbronchial lung biopsy
Abstract
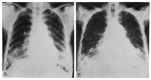
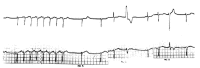
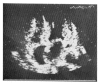
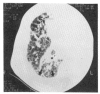
Diffuse alveolar septal involvement is a rare form of pulmonary amyloidosis. Antemortem diagnosis is unusual, and most of the reported cases were diagnosed at autopsy. It has recently been reported that transbronchial lung biopsy via a flexible fiberoptic bronchoscope was a relatively safe method to confirm diffuse alveolar septal amyloidosis. We report a case of pulmonary diffuse alveolar septal amyloidosis confirmed by transbronchial lung biopsy. The patient's chief complaints were dyspnea on exertion and epigastric pain aggravated over a one-year period, while a chest roentgenogram showed bilateral diffuse interstitial infiltration. This case also showed nephrotic syndrome, cardiac arrhythmia, congestive heart failure, a tingling sensation in both hands and multiple nodules in the gastrointestinal tracts, suggesting involvement of the kidney, heart, peripheral nerves and gastrointestinal tracts. We propose that when diffuse interstitial lung disease is present with systemic signs such as nephrotic syndrome or cardiac arrhythmia, amyloidosis should be considered as a possible diagnosis. Also, transbronchial lung biopsy may be a useful confirmative diagnostic tool.
Figures


References
-
- Glenner GG. Amyloid deposits and amyloidosis: The beta fibrillosis. N Engl J Med. 1980;302:1283–1292. 1333–1343. - PubMed
-
- Cohen AS, Shirahama T, Sipe JD, Skinner M. Amyloid proteins, precursors, mediator, and enhancer. Lab Invest. 1983;48:1–4. - PubMed
-
- Kyle RA, Greipp PR. Amyloidosis (AL): Clinical and laboratory features in 229 cases. Mayo Clin Proc. 1983;58:665–683. - PubMed
-
- Kanada DJ, Sharma OP. Long-term survival with diffuse interstitial pulmonary amyloidosis. Am J Med. 1979;67:879–882. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
