A randomized, double blind, placebo-controlled trial of pioglitazone in combination with riluzole in amyotrophic lateral sclerosis
- PMID: 22715372
- PMCID: PMC3371007
- DOI: 10.1371/journal.pone.0037885
A randomized, double blind, placebo-controlled trial of pioglitazone in combination with riluzole in amyotrophic lateral sclerosis
Abstract
Background: Pioglitazone, an oral anti-diabetic that stimulates the PPAR-gamma transcription factor, increased survival of mice with amyotrophic lateral sclerosis (ALS).
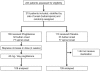
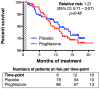
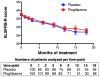
Methods/principal findings: We performed a phase II, double blind, multicentre, placebo controlled trial of pioglitazone in ALS patients under riluzole. 219 patients were randomly assigned to receive 45 mg/day of pioglitazone or placebo (one: one allocation ratio). The primary endpoint was survival. Secondary endpoints included incidence of non-invasive ventilation and tracheotomy, and slopes of ALS-FRS, slow vital capacity, and quality of life as assessed using EUROQoL EQ-5D. The study was conducted under a two-stage group sequential test, allowing to stop for futility or superiority after interim analysis. Shortly after interim analysis, 30 patients under pioglitazone and 24 patients under placebo had died. The trial was stopped for futility; the hazard ratio for primary endpoint was 1.21 (95% CI: 0.71-2.07, p = 0.48). Secondary endpoints were not modified by pioglitazone treatment. Pioglitazone was well tolerated.
Conclusion/significance: Pioglitazone has no beneficial effects on the survival of ALS patients as add-on therapy to riluzole.
Trial registration: Clinicaltrials.gov NCT00690118.
Conflict of interest statement
Figures
References
-
- Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996;347:1425–1431. - PubMed
-
- Philips T, Robberecht W. Neuroinflammation in amyotrophic lateral sclerosis: role of glial activation in motor neuron disease. Lancet Neurol. 2011;10:253–263. - PubMed
-
- Ceriello A. Thiazolidinediones as anti-inflammatory and anti-atherogenic agents. Diabetes Metab Res Rev. 2008;24:14–26. - PubMed
-
- Shibata N, Kawaguchi-Niida M, Yamamoto T, Toi S, Hirano A, et al. Effects of the PPARgamma activator pioglitazone on p38 MAP kinase and IkappaBalpha in the spinal cord of a transgenic mouse model of amyotrophic lateral sclerosis. Neuropathology. 2008;28:387–398. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

