Inhibition of fibroblast growth by Notch1 signaling is mediated by induction of Wnt11-dependent WISP-1
- PMID: 22715413
- PMCID: PMC3371022
- DOI: 10.1371/journal.pone.0038811
Inhibition of fibroblast growth by Notch1 signaling is mediated by induction of Wnt11-dependent WISP-1
Abstract
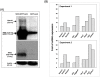
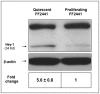
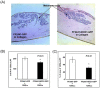
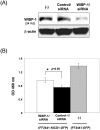
Fibroblasts are an integral component of stroma and important source of growth factors and extracellular matrix (ECM). They play a prominent role in maintaining tissue homeostasis and in wound healing and tumor growth. Notch signaling regulates biological function in a variety of cells. To elucidate the physiological function of Notch signaling in fibroblasts, we ablated Notch1 in mouse (Notch1(Flox/Flox)) embryonic fibroblasts (MEFs). Notch1-deficient (Notch1(-/-)) MEFs displayed faster growth and motility rate compared to Notch1(Flox/Flox) MEFs. Such phenotypic changes, however, were reversible by reconstitution of Notch1 activation via overexpression of the intracellular domain of Notch1 (NICD1) in Notch1-deficient MEFs. In contrast, constitutive activation of Notch1 signaling by introducing NICD1 into primary human dermal fibroblasts (FF2441), which caused pan-Notch activation, inhibited cell growth and motility, whereas cellular inhibition was relievable when the Notch activation was countered with dominant-negative mutant of Master-mind like 1 (DN-MAML-1). Functionally, "Notch-activated" stromal fibroblasts could inhibit tumor cell growth/invasion. Moreover, Notch activation induced expression of Wnt-induced secreted proteins-1 (WISP-1/CCN4) in FF2441 cells while deletion of Notch1 in MEFs resulted in an opposite effect. Notably, WISP-1 suppressed fibroblast proliferation, and was responsible for mediating Notch1's inhibitory effect since siRNA-mediated blockade of WISP-1 expression could relieve cell growth inhibition. Notch1-induced WISP-1 expression appeared to be Wnt11-dependent, but Wnt1-independent. Blockade of Wnt11 expression resulted in decreased WISP-1 expression and liberated Notch-induced cell growth inhibition. These findings indicated that inhibition of fibroblast proliferation by Notch pathway activation is mediated, at least in part, through regulating Wnt1-independent, but Wnt11-dependent WISP-1 expression.
Conflict of interest statement
Figures





Similar articles
-
Activation of Notch1 signaling in stromal fibroblasts inhibits melanoma growth by upregulating WISP-1.Oncogene. 2011 Oct 20;30(42):4316-26. doi: 10.1038/onc.2011.142. Epub 2011 Apr 25. Oncogene. 2011. PMID: 21516124
-
Notch1-WISP-1 axis determines the regulatory role of mesenchymal stem cell-derived stromal fibroblasts in melanoma metastasis.Oncotarget. 2016 Nov 29;7(48):79262-79273. doi: 10.18632/oncotarget.13021. Oncotarget. 2016. PMID: 27813493 Free PMC article.
-
Reprogramming of cancer-associated fibroblasts by apoptotic cancer cells inhibits lung metastasis via Notch1-WISP-1 signaling.Cell Mol Immunol. 2022 Dec;19(12):1373-1391. doi: 10.1038/s41423-022-00930-w. Epub 2022 Oct 14. Cell Mol Immunol. 2022. PMID: 36241874 Free PMC article.
-
Unlocking the therapeutic potential of WISP-1: A comprehensive exploration of its role in age-related musculoskeletal disorders.Int Immunopharmacol. 2025 Jan 3;145:113791. doi: 10.1016/j.intimp.2024.113791. Epub 2024 Dec 11. Int Immunopharmacol. 2025. PMID: 39667044 Review.
-
Notch1 in Cancer Therapy: Possible Clinical Implications and Challenges.Mol Pharmacol. 2020 Nov;98(5):559-576. doi: 10.1124/molpharm.120.000006. Epub 2020 Sep 10. Mol Pharmacol. 2020. PMID: 32913140 Review.
Cited by
-
Converting melanoma-associated fibroblasts into a tumor-suppressive phenotype by increasing intracellular Notch1 pathway activity.PLoS One. 2021 Mar 11;16(3):e0248260. doi: 10.1371/journal.pone.0248260. eCollection 2021. PLoS One. 2021. PMID: 33705467 Free PMC article.
-
Notch signaling regulates cell density-dependent apoptosis of NIH 3T3 through an IL-6/STAT3 dependent mechanism.Eur J Cell Biol. 2018 Sep;97(7):512-522. doi: 10.1016/j.ejcb.2018.09.001. Epub 2018 Sep 12. Eur J Cell Biol. 2018. PMID: 30249464 Free PMC article.
-
Mechanistic Interrogation of Cell Transformation In Vitro: The Transformics Assay as an Exemplar of Oncotransformation.Int J Mol Sci. 2022 Jul 9;23(14):7603. doi: 10.3390/ijms23147603. Int J Mol Sci. 2022. PMID: 35886950 Free PMC article.
-
Notch1 signaling determines the plasticity and function of fibroblasts in diabetic wounds.Life Sci Alliance. 2020 Oct 27;3(12):e202000769. doi: 10.26508/lsa.202000769. Print 2020 Dec. Life Sci Alliance. 2020. PMID: 33109684 Free PMC article.
-
Dual effect of WISP-1 in diverse pathological processes.Chin J Cancer Res. 2016 Dec;28(6):553-560. doi: 10.21147/j.issn.1000-9604.2016.06.01. Chin J Cancer Res. 2016. PMID: 28174483 Free PMC article.
References
-
- Becker V, Muller G. Biology of fibroblasts. Clinical and Experimental Nephrology. 1998;2:295–301.
-
- Ingber DE. Cancer as a disease of epithelial-mesenchymal interactions and extracellular matrix regulation. Differentiation. 2002;70:547–560. - PubMed
-
- Lynch CC, Matrisian LM. Matrix metalloproteinases in tumor-host cell communication. Differentiation. 2002;70:561–573. - PubMed
-
- Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol. 2004;36:1031–1037. - PubMed
-
- Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

