Moxifloxacin safety: an analysis of 14 years of clinical data
- PMID: 22715866
- PMCID: PMC3585838
- DOI: 10.2165/11634300-000000000-00000
Moxifloxacin safety: an analysis of 14 years of clinical data
Abstract
Background: Moxifloxacin, a fluoroquinolone antibiotic, is used for the treatment of respiratory tract, pelvic inflammatory disease, skin, and intra-abdominal infections. Its safety profile is considered favorable in most reviews but has been challenged with respect to rare but potentially fatal toxicities (e.g. hepatic, cardiac, or skin reactions).
Objective: To analyze and compare the safety profile of moxifloxacin versus comparators in the entire clinical database of the manufacturer.
Setting: Data on the valid-for-safety population from phase II-IV actively controlled studies (performed between 1996 and 2010) were analyzed. Studies were either double blind (n = 22 369) or open label (n = 7635) and included patients with indications that have been approved in at least one country [acute bacterial sinusitis, acute exacerbation of chronic bronchitis, community-acquired pneumonia, uncomplicated pelvic inflammatory disease, complicated and uncomplicated skin and skin structure infections, and complicated intra-abdominal infections] (n = 27 824) and patients with other indications (n = 2180), using the recommended daily dose (400 mg) and route of administration (oral, intravenous/oral, intravenous only). The analysis included patients at risk (age ≥65 years, diabetes mellitus, renal impairment, hepatic impairment, cardiac disorders, or body mass index <18 kg/m2). Patients with known contraindications were excluded from enrollment by study protocol design, but any patient having entered a study, even if inappropriately, was included in the analysis.
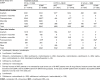
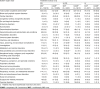
Main outcome measure: Crude incidences and relative risk estimates (Mantel-Haenszel analysis) of patients with any adverse event (AE), adverse drug reaction (ADR), serious AE (SAE), serious ADR (SADR), treatment discontinuation due to an AE or ADR, and fatal outcomes related to an AE or ADR.
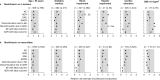
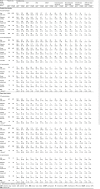
Results: Overall incidence rates of AEs were globally similar in the moxifloxacin and comparator groups. By filtering the data for differences in disfavor of moxifloxacin (i) at ≥2.5% for events with an incidence ≥2.5% or at ≥2-fold for events with an incidence <2.5% in one or both groups and (ii) affecting ≥10 patients in either group, we observed slightly more (i) AEs in double-blind intravenous-only and open-label oral studies, (ii) SAEs in double-blind intravenous-only studies, (iii) ADRs and SADRs in open-label oral studies, (iv) SADRs in open-label intravenous/oral studies, and (v) premature discontinuation due to AEs in open-label intravenous-only studies. The actual numbers of SADRs (in all studies) were small, with clinically relevant differences noted only in intravenous/oral studies and mainly driven by 'gastrointestinal disorders' (15 versus 7 patients) and 'changes observed during investigations' (23 versus 7 patients [asymptomatic QT prolongation: 11 versus 4 patients in double-blind studies]). Analysis by comparator (including another fluoroquinolone) did not reveal medically relevant differences, even in patients at risk. Incidence rates of hepatic disorders, tendon disorders, clinical surrogates of QT prolongation, serious cutaneous reactions, and Clostridium difficile-associated diarrhea were similar with moxifloxacin and comparators.
Conclusion: The safety of moxifloxacin is essentially comparable to that of standard therapies for patients receiving the currently registered dosage and for whom contraindications and precautions of use (as in the product label) are taken into account.
Figures










References
-
- Balter M.S., La Forge J., Low D.E., et al. Canadian guidelines for the management of acute exacerbations of chronic bronchitis. Can Respir J. 2003;10(B):3B–32B. - PubMed
-
- Solomkin J.S., Mazuski J.E., Bradley J.S., et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133–64. doi: 10.1086/649554. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

