Comparative vascular responses three months after paclitaxel and everolimus-eluting stent implantation in streptozotocin-induced diabetic porcine coronary arteries
- PMID: 22716997
- PMCID: PMC3413520
- DOI: 10.1186/1475-2840-11-75
Comparative vascular responses three months after paclitaxel and everolimus-eluting stent implantation in streptozotocin-induced diabetic porcine coronary arteries
Abstract
Background: Diabetes remains a significant risk factor for restenosis/thrombosis following stenting. Although vascular healing responses following drug-eluting stent (DES) treatment have been characterized previously in healthy animals, comparative assessments of different DES in a large animal model with isolated features of diabetes remains limited. We aimed to comparatively assess the vascular response to paclitaxel-eluting (PES) and everolimus-eluting (EES) stents in a porcine coronary model of streptozotocin (STZ)-induced type I diabetes.
Method: Twelve Yucatan swine were induced hyperglycemic with a single STZ dose intravenously to ablate pancreatic β-cells. After two months, each animal received one XIENCE V® (EES) and one Taxus Liberte (PES) stent, respectively, in each coronary artery. After three months, vascular healing was assessed by angiography and histomorphometry. Comparative in vitro effects of everolimus and paclitaxel (10-5 M-10-12 M) after 24 hours on carotid endothelial (EC) and smooth muscle (SMC) cell viability under hyperglycemic (42 mM) conditions were assayed by ELISA. Caspase-3 fluorescent assay was used to quantify caspase-3 activity of EC treated with everolimus or paclitaxel (10-5 M, 10-7 M) for 24 hours.
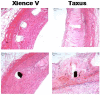
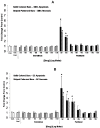
Results: After 3 months, EES reduced neointimal area (1.60 ± 0.41 mm, p < 0.001) with trends toward reduced % diameter stenosis (11.2 ± 9.8%, p = 0.12) and angiographic late-loss (0.28 ± 0.30 mm, p = 0.058) compared to PES (neointimal area: 2.74 ± 0.58 mm, % diameter stenosis: 19.3 ± 14.7%, late loss: 0.55 ± 0.53 mm). Histopathology revealed increased inflammation scores (0.54 ± 0.21 vs. 0.08 ± 0.05), greater medial necrosis grade (0.52 ± 0.26 vs. 0.0 ± 0.0), and persistently elevated fibrin scores (1.60 ± 0.60 vs. 0.63 ± 0.41) with PES compared to EES (p < 0.05). In vitro, paclitaxel significantly increased (p < 0.05) EC/SMC apoptosis/necrosis at high concentrations (≥ 10-7 M), while everolimus did not affect EC/SMC apoptosis/necrosis within the dose range tested. In ECs, paclitaxel (10-5 M) significantly increased caspase-3 activity (p < 0.05) while everolimus had no effect.
Conclusion: After 3 months, both DES exhibited signs of delayed healing in a STZ-induced diabetic swine model. PES exhibited greater neointimal area, increased inflammation, greater medial necrosis, and persistent fibrin compared to EES. Differential effects of everolimus and paclitaxel on vascular cell viability may potentially be a factor in regulating delayed healing observed with PES. Further investigation of molecular mechanisms may aid future development of stent-based therapies in treating coronary artery disease in diabetic patients.
Figures

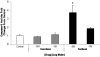
Similar articles
-
A clinical and angiographic study of the XIENCE V everolimus-eluting coronary stent system in the treatment of patients with multivessel coronary artery disease: the EXECUTIVE trial (EXecutive RCT: evaluating XIENCE V in a multi vessel disease).JACC Cardiovasc Interv. 2013 Oct;6(10):1012-22. doi: 10.1016/j.jcin.2013.05.016. Epub 2013 Sep 18. JACC Cardiovasc Interv. 2013. PMID: 24055444 Clinical Trial.
-
Intimal hyperplasia and vascular remodeling after everolimus-eluting and sirolimus-eluting stent implantation in diabetic patients: the randomized Diabetes and Drug-Eluting Stent (DiabeDES) IV Intravascular Ultrasound trial.Catheter Cardiovasc Interv. 2014 May 1;83(6):864-72. doi: 10.1002/ccd.25180. Epub 2013 Sep 30. Catheter Cardiovasc Interv. 2014. PMID: 23996918 Clinical Trial.
-
Meta-analysis of everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease: final 3-year results of the SPIRIT clinical trials program (Clinical Evaluation of the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients With De Novo Native Coronary Artery Lesions).JACC Cardiovasc Interv. 2013 Sep;6(9):914-22. doi: 10.1016/j.jcin.2013.05.005. JACC Cardiovasc Interv. 2013. PMID: 24050859
-
Recent progress in percutaneous coronary intervention: evolution of the drug-eluting stents, focus on the XIENCE V drug-eluting stent.Coron Artery Dis. 2010 Jan;21(1):46-56. doi: 10.1097/MCA.0b013e328333f550. Coron Artery Dis. 2010. PMID: 19952925 Review.
-
The multiple roles of chemokines in the mechanisms of stent biocompatibility.Cardiovasc Res. 2021 Sep 28;117(11):2299-2308. doi: 10.1093/cvr/cvaa072. Cardiovasc Res. 2021. PMID: 32196069 Review.
Cited by
-
Propofol protects against high glucose-induced endothelial adhesion molecules expression in human umbilical vein endothelial cells.Cardiovasc Diabetol. 2013 Jan 11;12:13. doi: 10.1186/1475-2840-12-13. Cardiovasc Diabetol. 2013. PMID: 23311470 Free PMC article.
-
Zotarolimus-eluting stent utilization in small-vessel coronary artery disease (ZEUS).Heart Vessels. 2014 Jan;29(1):29-34. doi: 10.1007/s00380-013-0327-0. Epub 2013 Feb 24. Heart Vessels. 2014. PMID: 23436214
-
Modeling and imaging cardiac sympathetic neurodegeneration in Parkinson's disease.Am J Nucl Med Mol Imaging. 2014 Mar 20;4(2):125-59. eCollection 2014. Am J Nucl Med Mol Imaging. 2014. PMID: 24753981 Free PMC article. Review.
-
Drug-coated balloon angioplasty for severe pulmonary vein stenosis resulting from cryoballoon ablation for atrial fibrillation.J Cardiol Cases. 2022 Mar 29;26(1):35-38. doi: 10.1016/j.jccase.2022.02.009. eCollection 2022 Jul. J Cardiol Cases. 2022. PMID: 35923534 Free PMC article.
-
Polymeric stent materials dysregulate macrophage and endothelial cell functions: implications for coronary artery stent.Int J Cardiol. 2014 Jul 1;174(3):688-95. doi: 10.1016/j.ijcard.2014.04.228. Epub 2014 Apr 25. Int J Cardiol. 2014. PMID: 24820736 Free PMC article.
References
-
- Hamamdzic D, Fenning RS, Patel D, Mohler ER, Orlova KA, Wright AC, Llano R, Keane MG, Shannon RP, Birnbaum MJ, Wilensky RL. Akt pathway is hypoactivated by synergistic actions of diabetes mellitus and hypercholesterolemia resulting in advanced coronary artery disease. Am J Physiol Heart Circ Physiol. 2010;299(3):H699–H706. doi: 10.1152/ajpheart.00071.2010. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

