Abnormal glucose tolerance and insulin secretion in pancreas-specific Tcf7l2-null mice
- PMID: 22717537
- PMCID: PMC6101215
- DOI: 10.1007/s00125-012-2600-7
Abnormal glucose tolerance and insulin secretion in pancreas-specific Tcf7l2-null mice
Abstract
Aims/hypothesis: Individuals carrying type 2 diabetes risk alleles in TCF7L2 display decreased beta cell levels of T cell factor 7 like-2 (TCF7L2) immunoreactivity, and impaired insulin secretion and beta cell sensitivity to glucagon-like peptide 1 (GLP-1). Here, we sought to determine whether selective deletion of Tcf7l2 in mouse pancreas impairs insulin release and glucose homeostasis.
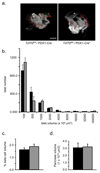
Methods: Pancreas-specific Tcf7l2-null (pTcf7l2) mice were generated by crossing mice carrying conditional knockout alleles of Tcf7l2 (Tcf7l2-flox) with mice expressing Cre recombinase under the control of the Pdx1 promoter (Pdx1.Cre). Gene expression was assessed by real-time quantitative PCR and beta cell mass by optical projection tomography. Glucose tolerance, insulin secretion from isolated islets, and plasma insulin, glucagon and GLP-1 content were assessed by standard protocols.
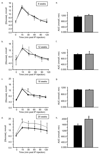
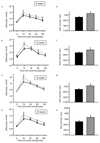
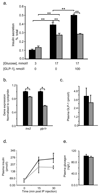
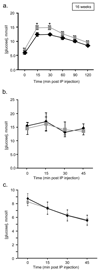
Results: From 12 weeks of age, pTcf7l2 mice displayed decreased oral glucose tolerance vs control littermates; from 20 weeks they had glucose intolerance upon administration of glucose by the intraperitoneal route. pTcf7l2 islets displayed impaired insulin secretion in response to 17 (vs 3.0) mmol/l glucose (54.6 ± 4.6%, p < 0.01) or to 17 mmol/l glucose plus 100 nmol/l GLP-1 (44.3 ± 4.9%, p < 0.01) compared with control islets. Glp1r (42 ± 0.08%, p < 0.01) and Ins2 (15.4 ± 4.6%, p < 0.01) expression was significantly lower in pTcf7l2 islets than in controls. Maintained on a high-fat (but not on a normal) diet, pTcf7l2 mice displayed decreased expansion of pancreatic beta cell volume vs control littermates. No differences were observed in plasma insulin, proinsulin, glucagon or GLP-1 concentrations.
Conclusions/interpretation: Selective deletion of Tcf7l2 in the pancreas replicates key aspects of the altered glucose homeostasis in human carriers of TCF7L2 risk alleles, indicating the direct role of this factor in controlling beta cell function.
Conflict of interest statement
The authors declare that there is no duality of interest associated with this manuscript.
Figures


Comment in
-
Impact of transcription factor 7-like 2 (TCF7L2) on pancreatic islet function and morphology in mice and men.Diabetologia. 2012 Oct;55(10):2559-2561. doi: 10.1007/s00125-012-2659-1. Epub 2012 Aug 5. Diabetologia. 2012. PMID: 22864463 Free PMC article.
References
-
- Pierce M, Keen H, Bradley C. Risk of diabetes in offspring of parents with non-insulin-dependent diabetes. Diabet Med. 1995;12:6–13. - PubMed
-
- Newman B, Selby JV, King MC, Slemenda C, Fabsitz R, Friedman GD. Concordance for type 2 (non-insulin-dependent) diabetes mellitus in male twins. Diabetologia. 1987;30:763–768. - PubMed
-
- Barroso I. Genetics of Type 2 diabetes. Diabet Med. 2005;22:517–535. - PubMed
-
- Sladek R, Rocheleau G, Rung J, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

