Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: an open-label trial
- PMID: 22717743
- PMCID: PMC4956470
- DOI: 10.1038/gt.2012.43
Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: an open-label trial
Abstract
We describe the 2-year follow-up of an open-label trial (CT-AMT-011-01) of AAV1-LPL(S447X) gene therapy for lipoprotein lipase (LPL) deficiency (LPLD), an orphan disease associated with chylomicronemia, severe hypertriglyceridemia, metabolic complications and potentially life-threatening pancreatitis. The LPL(S447X) gene variant, in an adeno-associated viral vector of serotype 1 (alipogene tiparvovec), was administered to 14 adult LPLD patients with a prior history of pancreatitis. Primary objectives were to assess the long-term safety of alipogene tiparvovec and achieve a ≥40% reduction in fasting median plasma triglyceride (TG) at 3-12 weeks compared with baseline. Cohorts 1 (n=2) and 2 (n=4) received 3 × 10(11) gc kg(-1), and cohort 3 (n=8) received 1 × 10(12) gc kg(-1). Cohorts 2 and 3 also received immunosuppressants from the time of alipogene tiparvovec administration and continued for 12 weeks. Alipogene tiparvovec was well tolerated, without emerging safety concerns for 2 years. Half of the patients demonstrated a ≥40% reduction in fasting TG between 3 and 12 weeks. TG subsequently returned to baseline, although sustained LPL(S447X) expression and long-term changes in TG-rich lipoprotein characteristics were noted independently of the effect on fasting plasma TG.
Trial registration: ClinicalTrials.gov NCT01109498.
Conflict of interest statement
The funding body (AMT) was involved in all aspects of the study (study design, data collection and analysis, and data interpretation in collaboration with the CRO and principal investigator. Five authors (J.dW., J.T., S.vD., N.vdB. and V.S-F) are employees of AMT. The remaining authors declare no conflict of interest. The principal investigator of the study (DG) has no financial interest in AMT and made all final editorial decisions regarding the manuscript.
Figures
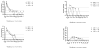




References
-
- Brunzell JD, Deeb SS. Familial lipoprotein lipase deficiency, Apo C-II deficiency and hepatic lipase deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic Basis of Inherited Disease. 8. New York, NY: McGraw-Hill; 2001. pp. 2789–2816.
-
- Black DM, Sprecher DL. Dietary treatment and growth of hyperchylomicronemic children severely restricted in dietary fat. Am J Dis Child. 1993;147:60–62. - PubMed
-
- Chait A, Robertson HT, Brunzell JD. Chylomicronemia syndrome in diabetes mellitus. Diabetes Care. 1981;4:343–348. - PubMed
-
- Chait A, Brunzell JD. Chylomicronemia syndrome. Adv Int Med. 1992;37:249–273. - PubMed
-
- Santamarina-Fojo S. The familial chylomicronemia syndrome. Endocrinol Metab Clin North Am. 1998;27:551–567. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

