Rules of engagement for base excision repair in chromatin
- PMID: 22718094
- PMCID: PMC3468691
- DOI: 10.1002/jcp.24134
Rules of engagement for base excision repair in chromatin
Abstract
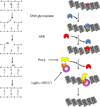
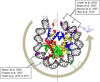
Most of the DNA in eukaryotes is packaged in tandemly arrayed nucleosomes that, together with numerous DNA- and nucleosome-associated enzymes and regulatory factors, make up chromatin. Chromatin modifying and remodeling agents help regulate access to selected DNA segments in chromatin, thereby facilitating transcription and DNA replication and repair. Studies of nucleotide excision repair (NER), single strand break repair (SSBR), and the homology-directed repair (HDR), and non-homologous end-joining (NHEJ) double strand break repair pathways have led to an "access-repair-restore" paradigm, in which chromatin in the vicinity of damaged DNA is disrupted, thereby enabling efficient repair and the subsequent repackaging of DNA into nucleosomes. When damage is extensive, these repair processes are accompanied by cell cycle checkpoint activation, which provides cells with sufficient time to either complete the repair or initiate apoptosis. It is not clear, however, if base excision repair (BER) of the ~20,000 or more oxidative DNA damages that occur daily in each nucleated human cell can be viewed through this same lens. Until recently, we did not know if BER requires or is accompanied by nucleosome disruption, and it is not yet clear that anything short of overwhelming oxidative damage (resulting in the shunting of DNA substrates into other repair pathways) results in checkpoint activation. This review highlights studies of how oxidatively damaged DNA in nucleosomes is discovered and repaired, and offers a working model of events associated with BER in chromatin that we hope will have heuristic value.
Copyright © 2012 Wiley Periodicals, Inc.
Figures


References
-
- Anderson JD, Widom J. Sequence and position-dependence of the equilibrium accessibility of nucleosomal DNA target sites. Journal of molecular biology. 2000;296(4):979–987. - PubMed
-
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual review of plant biology. 2004;55:373–399. - PubMed
-
- Banerjee A, Santos WL, Verdine GL. Structure of a DNA glycosylase searching for lesions. Science (New York, NY. 2006;311(5764):1153–1157. - PubMed
-
- Beard WA, Wilson SH. Structural design of a eukaryotic DNA repair polymerase: DNA polymerase beta. Mutation research. 2000;460(3–4):231–244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

