Absence of FKBP10 in recessive type XI osteogenesis imperfecta leads to diminished collagen cross-linking and reduced collagen deposition in extracellular matrix
- PMID: 22718341
- PMCID: PMC3470738
- DOI: 10.1002/humu.22139
Absence of FKBP10 in recessive type XI osteogenesis imperfecta leads to diminished collagen cross-linking and reduced collagen deposition in extracellular matrix
Abstract
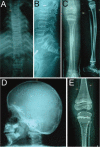
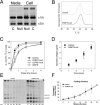
Recessive osteogenesis imperfecta (OI) is caused by defects in genes whose products interact with type I collagen for modification and/or folding. We identified a Palestinian pedigree with moderate and lethal forms of recessive OI caused by mutations in FKBP10 or PPIB, which encode endoplasmic reticulum resident chaperone/isomerases FKBP65 and CyPB, respectively. In one pedigree branch, both parents carry a deletion in PPIB (c.563_566delACAG), causing lethal type IX OI in their two children. In another branch, a child with moderate type XI OI has a homozygous FKBP10 mutation (c.1271_1272delCCinsA). Proband FKBP10 transcripts are 4% of control and FKBP65 protein is absent from proband cells. Proband collagen electrophoresis reveals slight band broadening, compatible with ≈10% over-modification. Normal chain incorporation, helix folding, and collagen T(m) support a minimal general collagen chaperone role for FKBP65. However, there is a dramatic decrease in collagen deposited in culture despite normal collagen secretion. Mass spectrometry reveals absence of hydroxylation of the collagen telopeptide lysine involved in cross-linking, suggesting that FKBP65 is required for lysyl hydroxylase activity or access to type I collagen telopeptide lysines, perhaps through its function as a peptidylprolyl isomerase. Proband collagen to organics ratio in matrix is approximately 30% of normal in Raman spectra. Immunofluorescence shows sparse, disorganized collagen fibrils in proband matrix.
Published 2012 Wiley Periodicals, Inc.*This article is a US Government work and, as such, is in the public domain of the United States of America.
Figures



References
-
- Arvan P, Zhao X, Ramos-Castaneda J, Chang A. Secretory pathway quality control operating in Golgi, plasmalemmal, and endosomal systems. Traffic. 2002;3:771–780. - PubMed
-
- Bailey AJ, Wotton SF, Sims TJ, Thompson PW. Biochemical changes in the collagen of human osteoporotic bone matrix. Connect Tissue Res. 1993;29:119–132. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

