Retinoic acid protects cardiomyocytes from high glucose-induced apoptosis through inhibition of NF-κB signaling pathway
- PMID: 22718360
- PMCID: PMC3470832
- DOI: 10.1002/jcp.24142
Retinoic acid protects cardiomyocytes from high glucose-induced apoptosis through inhibition of NF-κB signaling pathway
Abstract
We have previously shown that retinoic acid (RA) has protective effects on high glucose (HG)-induced cardiomyocyte apoptosis. To further elucidate the molecular mechanisms of RA effects, we determined the interaction between nuclear factor (NF)-κB and RA signaling. HG induced a sustained phosphorylation of IKK/IκBα and transcriptional activation of NF-κB in cardiomyocytes. Activated NF-κB signaling has an important role in HG-induced cardiomyocyte apoptosis and gene expression of interleukin-6 (IL-6), tumor necrosis factor (TNF)-α, and monocyte chemoattractant protein-1 (MCP-1). All-trans RA (ATRA) and LGD1069, through activation of RAR/RXR-mediated signaling, inhibited the HG-mediated effects in cardiomyocytes. The inhibitory effect of RA on NF-κB activation was mediated through inhibition of IKK/IκBα phosphorylation. ATRA and LGD1069 treatment promoted protein phosphatase 2A (PP2A) activity, which was significantly suppressed by HG stimulation. The RA effects on IKK and IκBα were blocked by okadaic acid or silencing the expression of PP2Ac-subunit, indicating that the inhibitory effect of RA on NF-κB is regulated through activation of PP2A and subsequent dephosphorylation of IKK/IκBα. Moreover, ATRA and LGD1069 reversed the decreased PP2A activity and inhibited the activation of IKK/IκBα and gene expression of MCP-1, IL-6, and TNF-α in the hearts of Zucker diabetic fatty rats. In summary, our findings suggest that the suppressed activation of PP2A contributed to sustained activation of NF-κB in HG-stimulated cardiomyocytes; and that the protective effect of RA on hyperglycemia-induced cardiomyocyte apoptosis and inflammatory responses is partially regulated through activation of PP2A and suppression of NF-κB-mediated signaling and downstream targets.
Copyright © 2012 Wiley Periodicals, Inc.
Figures


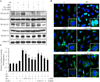


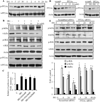


Similar articles
-
Atorvastatin Alleviates Experimental Diabetic Cardiomyopathy by Regulating the GSK-3β-PP2Ac-NF-κB Signaling Axis.PLoS One. 2016 Nov 16;11(11):e0166740. doi: 10.1371/journal.pone.0166740. eCollection 2016. PLoS One. 2016. PMID: 27851811 Free PMC article.
-
Retinoic acid receptor-mediated signaling protects cardiomyocytes from hyperglycemia induced apoptosis: role of the renin-angiotensin system.J Cell Physiol. 2011 May;226(5):1292-307. doi: 10.1002/jcp.22457. J Cell Physiol. 2011. PMID: 20945395 Free PMC article.
-
High-glucose-induced apoptosis, ROS production and pro-inflammatory response in cardiomyocytes is attenuated by metformin treatment via PP2A activation.J Biosci. 2020;45:126. J Biosci. 2020. PMID: 33184242
-
Differential signaling circuits in regulation of ultraviolet C light-induced early- and late-phase activation of NF-κB.Photochem Photobiol. 2010 Sep-Oct;86(5):995-9. doi: 10.1111/j.1751-1097.2010.00767.x. Photochem Photobiol. 2010. PMID: 20553411 Free PMC article. Review.
-
Overview of pyroptosis mechanism and in-depth analysis of cardiomyocyte pyroptosis mediated by NF-κB pathway in heart failure.Biomed Pharmacother. 2024 Oct;179:117367. doi: 10.1016/j.biopha.2024.117367. Epub 2024 Aug 29. Biomed Pharmacother. 2024. PMID: 39214011 Review.
Cited by
-
Loss of myocardial retinoic acid receptor α induces diastolic dysfunction by promoting intracellular oxidative stress and calcium mishandling in adult mice.J Mol Cell Cardiol. 2016 Oct;99:100-112. doi: 10.1016/j.yjmcc.2016.08.009. Epub 2016 Aug 15. J Mol Cell Cardiol. 2016. PMID: 27539860 Free PMC article.
-
All-trans-retinoic acid ameliorates doxorubicin-induced cardiotoxicity: in vivo potential involvement of oxidative stress, inflammation, and apoptosis via caspase-3 and p53 down-expression.Naunyn Schmiedebergs Arch Pharmacol. 2018 Jan;391(1):59-70. doi: 10.1007/s00210-017-1437-5. Epub 2017 Oct 30. Naunyn Schmiedebergs Arch Pharmacol. 2018. PMID: 29085977
-
Molecular Mechanisms of Retinoid Receptors in Diabetes-Induced Cardiac Remodeling.J Clin Med. 2014 Jun 4;3(2):566-94. doi: 10.3390/jcm3020566. J Clin Med. 2014. PMID: 26237391 Free PMC article. Review.
-
Retinol intake is associated with the risk of chronic kidney disease in individuals with type 2 diabetes mellitus: results from NHANES.Sci Rep. 2023 Jul 18;13(1):11567. doi: 10.1038/s41598-023-38582-z. Sci Rep. 2023. PMID: 37463986 Free PMC article.
-
All-trans retinoic acid protects against doxorubicin-induced cardiotoxicity by activating the ERK2 signalling pathway.Br J Pharmacol. 2016 Jan;173(2):357-71. doi: 10.1111/bph.13377. Epub 2015 Dec 19. Br J Pharmacol. 2016. PMID: 26507774 Free PMC article.
References
-
- Anest V, Hanson JL, Cogswell PC, Steinbrecher KA, Strahl BD, Baldwin AS. A nucleosomal function for IkappaB kinase-alpha in NF-kappaB-dependent gene expression. Nature. 2003;423(6940):659–663. - PubMed
-
- Aso Y, Okumura K, Yoshida N, Tayama K, Kanda T, Kobayashi I, Takemura Y, Inukai T. Plasma interleukin-6 is associated with coagulation in poorly controlled patients with Type 2 diabetes. Diabet Med. 2003;20(11):930–934. - PubMed
-
- Austenaa LM, Carlsen H, Ertesvag A, Alexander G, Blomhoff HK, Blomhoff R. Vitamin A status significantly alters nuclear factor-kappaB activity assessed by in vivo imaging. FASEB J. 2004;18(11):1255–1257. - PubMed
-
- Baena RM, Campoy C, Bayes R, Blanca E, Fernandez JM, Molina-Font JA. Vitamin A retinol binding protein and lipids in type 1 diabetes mellitus. Eur J Clin Nutr. 2002;56(1):44–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

