The rates of Ca2+ dissociation and cross-bridge detachment from ventricular myofibrils as reported by a fluorescent cardiac troponin C
- PMID: 22718768
- PMCID: PMC3431663
- DOI: 10.1074/jbc.M111.337295
The rates of Ca2+ dissociation and cross-bridge detachment from ventricular myofibrils as reported by a fluorescent cardiac troponin C
Abstract
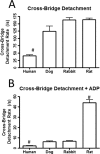
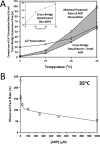
The rate-limiting step of cardiac muscle relaxation has been proposed to reside in the myofilament. Both the rates of cross-bridge detachment and Ca(2+) dissociation from troponin C (TnC) have been hypothesized to rate-limit myofilament inactivation. In this study we used a fluorescent TnC to measure both the rate of Ca(2+) dissociation from TnC and the rate of cross-bridge detachment from several different species of ventricular myofibrils. The fluorescently labeled TnC was sensitive to both Ca(2+) dissociation and cross-bridge detachment at low Ca(2+) (presence of EGTA), allowing for a direct comparison between the two proposed rates of myofilament inactivation. Unlike Ca(2+) dissociation from TnC, cross-bridge detachment varied in myofibrils from different species and was rate-limited by ADP release. At subphysiological temperatures (<20 °C), the rate of Ca(2+) dissociation from TnC was faster than the rate of cross-bridge detachment in the presence of ADP. These results support the hypothesis that cross-bridge detachment rate-limits relaxation. However, Ca(2+) dissociation from TnC was not as temperature-sensitive as cross-bridge detachment. At a near physiological temperature (35 °C) and ADP, the rate of cross-bridge detachment may actually be faster than the rate of Ca(2+) dissociation. This provides evidence that there may not be a simple, single rate-limiting step of myofilament inactivation.
Figures





References
-
- Grossman W. (1990) Diastolic function and heart failure. An overview. Eur. Heart J. 11, 2–7 - PubMed
-
- Bers D. M. (2002) Cardiac excitation-contraction coupling. Nature 415, 198–205 - PubMed
-
- Janssen P. M., Stull L. B., Marbán E. (2002) Myofilament properties comprise the rate-limiting step for cardiac relaxation at body temperature in the rat. Am. J. Physiol. Heart Circ. Physiol. 282, H499–H507 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

