Roles of cell signaling pathways in cell-to-cell contact-mediated Epstein-Barr virus transmission
- PMID: 22718812
- PMCID: PMC3416120
- DOI: 10.1128/JVI.00712-12
Roles of cell signaling pathways in cell-to-cell contact-mediated Epstein-Barr virus transmission
Abstract
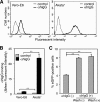
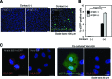
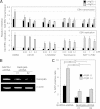
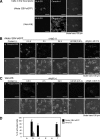
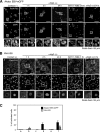
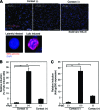
Epstein-Barr virus (EBV), a human gamma herpesvirus, establishes a life-long latent infection in B lymphocytes and epithelial cells following primary infection. Several lines of evidence indicate that the efficiency of EBV infection in epithelial cells is accelerated up to 10(4)-fold by coculturing with EBV-infected Burkitt's lymphoma (BL) cells compared to infection with cell-free virions, indicating that EBV infection into epithelial cells is mainly mediated via cell-to-cell contact. However, the molecular mechanisms involved in this pathway are poorly understood. Here, we establish a novel assay to assess cell-to-cell contact-mediated EBV transmission by coculturing an EBV-infected BL cell line with an EBV-negative epithelial cell line under stimulation for lytic cycle induction. By using this assay, we confirmed that EBV was transmitted from BL cells to epithelial cells via cell-to-cell contact but not via cell-to-cell fusion. The inhibitor treatments of extracellular signal-regulated kinase (ERK) and nuclear factor (NF)-κB pathways blocked EBV transmission in addition to lytic induction. The blockage of the phosphoinositide 3-kinase (PI3K) pathway impaired EBV transmission coupled with the inhibition of lytic induction. Knockdown of the RelA/p65 subunit of NF-κB reduced viral transmission. Moreover, these signaling pathways were activated in cocultured BL cells and in epithelial cells. Finally, we observed that viral replication was induced in cocultured BL cells. Taken together, our data suggest that cell-to-cell contact induces multiple cell signaling pathways in BL cells and epithelial cells, contributing to the induction of the viral lytic cycle in BL cells and the enhancement of viral transmission to epithelial cells.
Figures


Similar articles
-
Epstein-Barr virus exploits host endocytic machinery for cell-to-cell viral transmission rather than a virological synapse.J Gen Virol. 2016 Nov;97(11):2989-3006. doi: 10.1099/jgv.0.000605. Epub 2016 Sep 19. J Gen Virol. 2016. PMID: 27655016
-
Interferon-γ-inducible protein 16 (IFI16) is required for the maintenance of Epstein-Barr virus latency.Virol J. 2017 Nov 13;14(1):221. doi: 10.1186/s12985-017-0891-5. Virol J. 2017. PMID: 29132393 Free PMC article.
-
IRF4 promotes Epstein-Barr virus activation in Burkitt's lymphoma cells.J Gen Virol. 2019 May;100(5):851-862. doi: 10.1099/jgv.0.001249. Epub 2019 Mar 25. J Gen Virol. 2019. PMID: 30907723
-
EBV Infection and Glucose Metabolism in Nasopharyngeal Carcinoma.Adv Exp Med Biol. 2017;1018:75-90. doi: 10.1007/978-981-10-5765-6_6. Adv Exp Med Biol. 2017. PMID: 29052133 Review.
-
Epstein-Barr virus latency: LMP2, a regulator or means for Epstein-Barr virus persistence?Adv Cancer Res. 2000;79:175-200. doi: 10.1016/s0065-230x(00)79006-3. Adv Cancer Res. 2000. PMID: 10818681 Review.
Cited by
-
Significance of Interviral Recombination as Novel Mechanism for Extending Viral Disease Repertoire.Brain Disord Ther. 2016 Oct;5(3):127. doi: 10.4172/2168-975X.1000217. Epub 2016 Jun 23. Brain Disord Ther. 2016. PMID: 28018769 Free PMC article.
-
Exosomes derived from Epstein-Barr virus-infected cells are internalized via caveola-dependent endocytosis and promote phenotypic modulation in target cells.J Virol. 2013 Sep;87(18):10334-47. doi: 10.1128/JVI.01310-13. Epub 2013 Jul 17. J Virol. 2013. PMID: 23864627 Free PMC article.
-
Fascin in Gynecological Cancers: An Update of the Literature.Cancers (Basel). 2021 Nov 17;13(22):5760. doi: 10.3390/cancers13225760. Cancers (Basel). 2021. PMID: 34830909 Free PMC article. Review.
-
The tumor marker Fascin is induced by the Epstein-Barr virus-encoded oncoprotein LMP1 via NF-κB in lymphocytes and contributes to their invasive migration.Cell Commun Signal. 2014 Jul 11;12:46. doi: 10.1186/s12964-014-0046-x. Cell Commun Signal. 2014. PMID: 25105941 Free PMC article.
-
Hsa-miR-7974 Suppresses Epstein-Barr Virus Reactivation by Directly Targeting BZLF1 and BRLF1.Viruses. 2025 Apr 23;17(5):594. doi: 10.3390/v17050594. Viruses. 2025. PMID: 40431607 Free PMC article.
References
-
- Akiyama S, et al. 1988. Characteristics of three human gastric cancer cell lines, NU-GC-2, NU-GC-3 and NU-GC-4. Jpn. J. Surg. 18:438–446 - PubMed
-
- Barranco SC, et al. 1983. Establishment and characterization of an in vitro model system for human adenocarcinoma of the stomach. Cancer Res. 43:1703–1709 - PubMed
-
- Boulton TG, et al. 1991. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell 65:663–675 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

