Proteome-wide overexpression of host proteins for identification of factors affecting tombusvirus RNA replication: an inhibitory role of protein kinase C
- PMID: 22718827
- PMCID: PMC3416130
- DOI: 10.1128/JVI.00019-12
Proteome-wide overexpression of host proteins for identification of factors affecting tombusvirus RNA replication: an inhibitory role of protein kinase C
Abstract
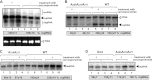
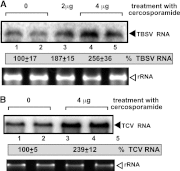
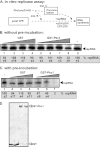
To identify host genes affecting replication of Tomato bushy stunt virus (TBSV), a small model positive-stranded RNA virus, we overexpressed 5,500 yeast proteins individually in Saccharomyces cerevisiae, which supports TBSV replication. In total, we identified 141 host proteins, and overexpression of 40 of those increased and the remainder decreased the accumulation of a TBSV replicon RNA. Interestingly, 36 yeast proteins were identified previously by various screens, greatly strengthening the relevance of these host proteins in TBSV replication. To validate the results from the screen, we studied the effect of protein kinase C1 (Pkc1), a conserved host kinase involved in many cellular processes, which inhibited TBSV replication when overexpressed. Using a temperature-sensitive mutant of Pkc1p revealed a high level of TBSV replication at a semipermissive temperature, further supporting the idea that Pkc1p is an inhibitor of TBSV RNA replication. A direct inhibitory effect of Pkc1p was shown in a cell-free yeast extract-based TBSV replication assay, in which Pkc1p likely phosphorylates viral replication proteins, decreasing their abilities to bind to the viral RNA. We also show that cercosporamide, a specific inhibitor of Pkc-like kinases, leads to increased TBSV replication in yeast, in plant single cells, and in whole plants, suggesting that Pkc-related pathways are potent inhibitors of TBSV in several hosts.
Figures


References
-
- Ali A, Sivakami S, Raghuram N. 2007. Regulation of activity and transcript levels of NR in rice (Oryza sativa): roles of protein kinase and G-proteins. Plant Science 172:406–413
-
- Barajas D, Jiang Y, Nagy PD. 2009. A unique role for the host ESCRT proteins in replication of tomato bushy stunt virus. PLoS Pathog. 5:e1000705 doi:10.1371/journal.ppat.1000705 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

