Inhibition of human cytomegalovirus immediate-early gene expression by cyclin A2-dependent kinase activity
- PMID: 22718829
- PMCID: PMC3416125
- DOI: 10.1128/JVI.07181-11
Inhibition of human cytomegalovirus immediate-early gene expression by cyclin A2-dependent kinase activity
Abstract
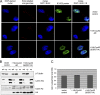
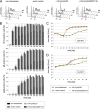
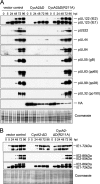
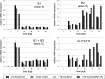
Human cytomegalovirus (HCMV) starts its lytic replication cycle only in the G(0)/G(1) phase of the cell division cycle. S/G(2) cells can be infected but block the onset of immediate-early (IE) gene expression. This block can be overcome by inhibition of cyclin-dependent kinases (CDKs), suggesting that cyclin A2, the only cyclin with an S/G(2)-specific activity profile, may act as a negative regulator of viral gene expression. To directly test this hypothesis, we generated derivatives of an HCMV-permissive glioblastoma cell line that express cyclin A2 in a constitutive, cell cycle-independent manner. We demonstrate that even moderate cyclin A2 overexpression in G(1) was sufficient to severely compromise the HCMV replicative cycle after high-multiplicity infection. This negative effect was composed of a strong but transient inhibition of IE gene transcription and a more sustained alteration of IE mRNA processing, resulting in reduced levels of UL37 and IE2, an essential transactivator of viral early gene expression. Consistently, cyclin A2-overexpressing cells showed a strong delay of viral early and late gene expression, as well as virus reproduction. All effects were dependent on CDK activity, as a cyclin A2 mutant deficient in CDK binding was unable to interfere with the HCMV infectious cycle. Interestingly, murine CMV, whose IE gene expression is known to be cell cycle independent, is not affected by cyclin A2. Instead, it upregulates cyclin A2-associated kinase activity upon infection. Understanding the mechanisms behind the HCMV-specific action of cyclin A2-CDK might reveal new targets for antiviral strategies.
Figures




Similar articles
-
Human cytomegalovirus tegument protein pp150 acts as a cyclin A2-CDK-dependent sensor of the host cell cycle and differentiation state.Proc Natl Acad Sci U S A. 2013 Oct 22;110(43):17510-5. doi: 10.1073/pnas.1312235110. Epub 2013 Oct 7. Proc Natl Acad Sci U S A. 2013. PMID: 24101496 Free PMC article.
-
PUL21a-Cyclin A2 interaction is required to protect human cytomegalovirus-infected cells from the deleterious consequences of mitotic entry.PLoS Pathog. 2014 Nov 13;10(10):e1004514. doi: 10.1371/journal.ppat.1004514. eCollection 2014 Oct. PLoS Pathog. 2014. PMID: 25393019 Free PMC article.
-
Cyclin-dependent kinase activity is required at early times for accurate processing and accumulation of the human cytomegalovirus UL122-123 and UL37 immediate-early transcripts and at later times for virus production.J Virol. 2004 Oct;78(20):11219-32. doi: 10.1128/JVI.78.20.11219-11232.2004. J Virol. 2004. PMID: 15452241 Free PMC article.
-
Human cytomegalovirus riding the cell cycle.Med Microbiol Immunol. 2015 Jun;204(3):409-19. doi: 10.1007/s00430-015-0396-z. Epub 2015 Mar 17. Med Microbiol Immunol. 2015. PMID: 25776080 Review.
-
Inhibition of cytomegalovirus immediate early gene expression: a therapeutic option?Antiviral Res. 2001 Mar;49(3):129-45. doi: 10.1016/s0166-3542(01)00126-7. Antiviral Res. 2001. PMID: 11428240 Review.
Cited by
-
SARS-CoV-2 encoded ORF3a interacts with YY1 to promote latent HCMV reactivation.PLoS Pathog. 2025 Jul 16;21(7):e1013344. doi: 10.1371/journal.ppat.1013344. eCollection 2025 Jul. PLoS Pathog. 2025. PMID: 40668819 Free PMC article.
-
MicroRNA miR-21 attenuates human cytomegalovirus replication in neural cells by targeting Cdc25a.J Virol. 2015 Jan 15;89(2):1070-82. doi: 10.1128/JVI.01740-14. Epub 2014 Nov 5. J Virol. 2015. PMID: 25378484 Free PMC article.
-
The host ubiquitin-dependent segregase VCP/p97 is required for the onset of human cytomegalovirus replication.PLoS Pathog. 2017 May 11;13(5):e1006329. doi: 10.1371/journal.ppat.1006329. eCollection 2017 May. PLoS Pathog. 2017. PMID: 28494016 Free PMC article.
-
Activation of E2F-dependent transcription by the mouse cytomegalovirus M117 protein affects the viral host range.PLoS Pathog. 2018 Dec 10;14(12):e1007481. doi: 10.1371/journal.ppat.1007481. eCollection 2018 Dec. PLoS Pathog. 2018. PMID: 30532172 Free PMC article.
-
Cyclin A degradation by primate cytomegalovirus protein pUL21a counters its innate restriction of virus replication.PLoS Pathog. 2013;9(12):e1003825. doi: 10.1371/journal.ppat.1003825. Epub 2013 Dec 26. PLoS Pathog. 2013. PMID: 24385906 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

