Characterization in vitro and in vivo of pandemic (H1N1) 2009 influenza viruses isolated from patients
- PMID: 22718834
- PMCID: PMC3416174
- DOI: 10.1128/JVI.01214-12
Characterization in vitro and in vivo of pandemic (H1N1) 2009 influenza viruses isolated from patients
Abstract
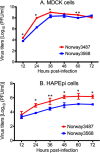
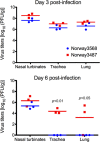
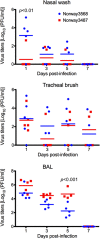
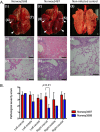
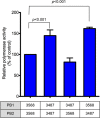
The first influenza pandemic of the 21st century was caused by novel H1N1 viruses that emerged in early 2009. Molecular evolutionary analyses of the 2009 pandemic influenza A H1N1 [A(H1N1)pdm09] virus revealed two major clusters, cluster I and cluster II. Although the pathogenicity of viruses belonging to cluster I, which became extinct by the end of 2009, has been examined in a nonhuman primate model, the pathogenic potential of viruses belonging to cluster II, which has spread more widely in the world, has not been studied in this animal model. Here, we characterized two Norwegian isolates belonging to cluster II, namely, A/Norway/3568/2009 (Norway3568) and A/Norway/3487-2/2009 (Norway3487), which caused distinct clinical symptoms, despite their genetic similarity. We observed more efficient replication in cultured cells and delayed virus clearance from ferret respiratory organs for Norway3487 virus, which was isolated from a severe case, compared with the efficiency of replication and time of clearance of Norway3568 virus, which was isolated from a mild case. Moreover, Norway3487 virus to some extent caused more severe lung damage in nonhuman primates than did Norway3568 virus. Our data suggest that the distinct replicative and pathogenic potentials of these two viruses may result from differences in their biological properties (e.g., the receptor-binding specificity of hemagglutinin and viral polymerase activity).
Figures


References
-
- Bautista E, et al. 2010. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N. Engl. J. Med. 362:1708–1719 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

