A Nuclear localization signal in herpesvirus protein VP1-2 is essential for infection via capsid routing to the nuclear pore
- PMID: 22718835
- PMCID: PMC3416164
- DOI: 10.1128/JVI.01209-12
A Nuclear localization signal in herpesvirus protein VP1-2 is essential for infection via capsid routing to the nuclear pore
Abstract
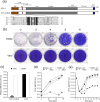
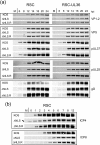
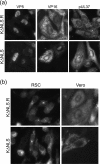
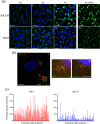
To initiate infection, herpesviruses must navigate to and transport their genomes across the nuclear pore. VP1-2 is a large structural protein of the virion that is conserved in all herpesviruses and plays multiple essential roles in virus replication, including roles in early entry. VP1-2 contains an N-terminal basic motif which functions as an efficient nuclear localization signal (NLS). In this study, we constructed a mutant HSV strain, K.VP1-2ΔNLS, which contains a 7-residue deletion of the core NLS at position 475. This mutant fails to spread in normal cells but can be propagated in complementing cell lines. Electron microscopy (EM) analysis of infection in noncomplementing cells demonstrated capsid assembly, cytoplasmic envelopment, and the formation of extracellular enveloped virions. Furthermore, extracellular virions isolated from noncomplementing cells had similar profiles and abundances of structural proteins. Virions containing VP1-2ΔNLS were able to enter and be transported within cells. However, further progress of infection was prevented, with at least a 500- to 1,000-fold reduction in the efficiency of initiating gene expression compared to that in the revertant. Ultrastructural and immunofluorescence analyses revealed that the K.VP1-2ΔNLS mutant was blocked at the microtubule organizing center or immediately upstream of nuclear pore docking and prior to gene expression. These results indicate that the VP1-2 NLS is not required for the known assembly functions of the protein but is a key requirement for the early routing to the nuclear pore that is necessary for successful infection. Given its conservation, we propose that this motif may also be critical for entry of other classes of herpesviruses.
Figures





References
-
- Abaitua F, Souto RN, Browne H, Daikoku T, O'Hare P. 2009. Characterization of the herpes simplex virus (HSV)-1 tegument protein VP1-2 during infection with the HSV temperature-sensitive mutant tsB7. J. Gen. Virol. 90:2353–2363 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

