Characterization of an apical ceramide-enriched compartment regulating ciliogenesis
- PMID: 22718902
- PMCID: PMC3418310
- DOI: 10.1091/mbc.E12-02-0079
Characterization of an apical ceramide-enriched compartment regulating ciliogenesis
Abstract
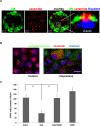
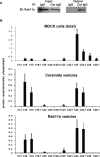
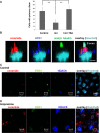
We show that in Madin-Darby canine kidney (MDCK) cells, an apical ceramide-enriched compartment (ACEC) at the base of primary cilia is colocalized with Rab11a. Ceramide and Rab11a vesicles isolated by magnetic sorting contain a highly similar profile of proteins (atypical protein kinase C [aPKC], Cdc42, Sec8, Rab11a, and Rab8) and ceramide species, suggesting the presence of a ciliogenic protein complex associated with ceramide at the ACEC. It is intriguing that C16 and C18 ceramide, although less abundant ceramide species in MDCK cells, are highly enriched in ceramide and Rab11a vesicles. Expression of a ceramide-binding but dominant-negative mutant of aPKC suppresses ciliogenesis, indicating that the association of ceramide with aPKC is critical for the formation of this complex. Our results indicate that ciliogenic ceramide is derived from apical sphingomyelin (SM) that is endocytosed and then converted to the ACEC. Consistently, inhibition of acid sphingomyelinase with imipramine disrupts ACEC formation, association of ciliogenic proteins with Rab11a vesicles, and cilium formation. Ciliogenesis is rescued by the histone deacetylase (HDAC) inhibitor trichostatin A, indicating that ceramide promotes tubulin acetylation in cilia. Taken together, our results suggest that the ACEC is a novel compartment in which SM-derived ceramide induces formation of a ciliogenic lipid-protein complex that sustains primary cilia by preventing deacetylation of microtubules.
Figures





Similar articles
-
Primary cilia in stem cells and neural progenitors are regulated by neutral sphingomyelinase 2 and ceramide.Mol Biol Cell. 2014 Jun;25(11):1715-29. doi: 10.1091/mbc.E13-12-0730. Epub 2014 Apr 2. Mol Biol Cell. 2014. PMID: 24694597 Free PMC article.
-
Regulation of primary cilia formation by ceramide.J Lipid Res. 2009 Oct;50(10):2103-10. doi: 10.1194/jlr.M900097-JLR200. Epub 2009 Apr 16. J Lipid Res. 2009. PMID: 19372594 Free PMC article.
-
Palmitoylation of acetylated tubulin and association with ceramide-rich platforms is critical for ciliogenesis.J Lipid Res. 2021;62:100021. doi: 10.1194/jlr.RA120001190. Epub 2021 Jan 7. J Lipid Res. 2021. PMID: 33380429 Free PMC article.
-
Rabs and other small GTPases in ciliary transport.Biol Cell. 2011 May;103(5):209-21. doi: 10.1042/BC20100150. Biol Cell. 2011. PMID: 21488838 Review.
-
Histone deacetylase inhibitors that target tubulin.Cancer Lett. 2009 Aug 8;280(2):222-32. doi: 10.1016/j.canlet.2009.01.040. Epub 2009 Mar 5. Cancer Lett. 2009. PMID: 19268440 Review.
Cited by
-
Epigenetic Regulation Mediated by Sphingolipids in Cancer.Int J Mol Sci. 2023 Mar 10;24(6):5294. doi: 10.3390/ijms24065294. Int J Mol Sci. 2023. PMID: 36982369 Free PMC article. Review.
-
The Neutral Sphingomyelinase 2 Is Required to Polarize and Sustain T Cell Receptor Signaling.Front Immunol. 2018 Apr 18;9:815. doi: 10.3389/fimmu.2018.00815. eCollection 2018. Front Immunol. 2018. PMID: 29720981 Free PMC article.
-
Sphingolipids and lipid rafts: Novel concepts and methods of analysis.Chem Phys Lipids. 2018 Nov;216:114-131. doi: 10.1016/j.chemphyslip.2018.08.003. Epub 2018 Sep 5. Chem Phys Lipids. 2018. PMID: 30194926 Free PMC article. Review.
-
Role of lipids in the control of autophagy and primary cilium signaling in neurons.Neural Regen Res. 2024 Feb;19(2):264-271. doi: 10.4103/1673-5374.377414. Neural Regen Res. 2024. PMID: 37488876 Free PMC article. Review.
-
Acid ceramidase inhibition ameliorates α-synuclein accumulation upon loss of GBA1 function.Hum Mol Genet. 2018 Jun 1;27(11):1972-1988. doi: 10.1093/hmg/ddy105. Hum Mol Genet. 2018. PMID: 29579237 Free PMC article.
References
-
- Albouz S, Le Saux F, Wenger D, Hauw JJ, Baumann N. Modifications of sphingomyelin and phosphatidylcholine metabolism by tricyclic antidepressants and phenothiazines. Life Sci. 1986;38:357–363. - PubMed
-
- Bieberich E, Hu B, Silva J, MacKinnon S, Yu RK, Fillmore H, Broaddus WC, Ottenbrite RM. Synthesis and characterization of novel ceramide analogs for induction of apoptosis in human cancer cells. Cancer Lett. 2002;181:55–64. - PubMed
-
- Bieberich E, Kawaguchi T, Yu RK. N-acylated serinol is a novel ceramide mimic inducing apoptosis in neuroblastoma cells. J Biol Chem. 2000;275:177–181. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

