Knockdown of ttc26 disrupts ciliogenesis of the photoreceptor cells and the pronephros in zebrafish
- PMID: 22718903
- PMCID: PMC3418303
- DOI: 10.1091/mbc.E12-01-0019
Knockdown of ttc26 disrupts ciliogenesis of the photoreceptor cells and the pronephros in zebrafish
Abstract
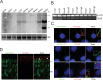
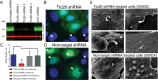
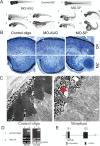
In our effort to understand genetic disorders of the photoreceptor cells of the retina, we have focused on intraflagellar transport in photoreceptor sensory cilia. From previous mouse proteomic data we identified a cilia protein Ttc26, orthologue of dyf-13 in Caenorhabditis elegans, as a target. We localized Ttc26 to the transition zone of photoreceptor and to the transition zone of cilia in cultured murine inner medullary collecting duct 3 (mIMCD3) renal cells. Knockdown of Ttc26 in mIMCD3 cells produced shortened and defective primary cilia, as revealed by immunofluorescence and scanning electron microscopy. To study Ttc26 function in sensory cilia in vivo, we utilized a zebrafish vertebrate model system. Morpholino knockdown of ttc26 in zebrafish embryos caused ciliary defects in the pronephric kidney at 27 h postfertilization and distension/dilation of pronephros at 5 d postfertilization (dpf). In the eyes, the outer segments of photoreceptor cells appeared shortened or absent, whereas cellular lamination appeared normal in retinas at 5 dpf. This suggests that loss of ttc26 function prevents normal ciliogenesis and differentiation in the photoreceptor cells, and that ttc26 is required for normal development and differentiation in retina and pronephros. Our studies support the importance of Ttc26 function in ciliogenesis and suggest that screening for TTC26 mutations in human ciliopathies is justified.
Figures


Similar articles
-
Knockdown of poc1b causes abnormal photoreceptor sensory cilium and vision impairment in zebrafish.Biochem Biophys Res Commun. 2015 Oct 2;465(4):651-7. doi: 10.1016/j.bbrc.2015.06.083. Epub 2015 Jul 15. Biochem Biophys Res Commun. 2015. PMID: 26188096 Free PMC article.
-
TTC26/DYF13 is an intraflagellar transport protein required for transport of motility-related proteins into flagella.Elife. 2014 Jan 1;3:e01566. doi: 10.7554/eLife.01566. Elife. 2014. PMID: 24596149 Free PMC article.
-
Sept6 is required for ciliogenesis in Kupffer's vesicle, the pronephros, and the neural tube during early embryonic development.Mol Cell Biol. 2014 Apr;34(7):1310-21. doi: 10.1128/MCB.01409-13. Epub 2014 Jan 27. Mol Cell Biol. 2014. PMID: 24469395 Free PMC article.
-
Kinesin-2 family motors in the unusual photoreceptor cilium.Vision Res. 2012 Dec 15;75:33-6. doi: 10.1016/j.visres.2012.10.008. Epub 2012 Oct 31. Vision Res. 2012. PMID: 23123805 Free PMC article. Review.
-
Zebrafish: a model system for the study of vertebrate renal development, function, and pathophysiology.Curr Opin Nephrol Hypertens. 2011 Jul;20(4):416-24. doi: 10.1097/MNH.0b013e3283477797. Curr Opin Nephrol Hypertens. 2011. PMID: 21519251 Review.
Cited by
-
Characterization of tetratricopeptide repeat-containing proteins critical for cilia formation and function.PLoS One. 2015 Apr 10;10(4):e0124378. doi: 10.1371/journal.pone.0124378. eCollection 2015. PLoS One. 2015. PMID: 25860617 Free PMC article.
-
A mutation in the mouse ttc26 gene leads to impaired hedgehog signaling.PLoS Genet. 2014 Oct 23;10(10):e1004689. doi: 10.1371/journal.pgen.1004689. eCollection 2014 Oct. PLoS Genet. 2014. PMID: 25340710 Free PMC article.
-
The role of primary cilia in the development and disease of the retina.Organogenesis. 2014 Jan 1;10(1):69-85. doi: 10.4161/org.26710. Epub 2013 Oct 25. Organogenesis. 2014. PMID: 24162842 Free PMC article. Review.
-
Active transport and diffusion barriers restrict Joubert Syndrome-associated ARL13B/ARL-13 to an Inv-like ciliary membrane subdomain.PLoS Genet. 2013;9(12):e1003977. doi: 10.1371/journal.pgen.1003977. Epub 2013 Dec 5. PLoS Genet. 2013. PMID: 24339792 Free PMC article.
-
EFEMP1 rare variants cause familial juvenile-onset open-angle glaucoma.Hum Mutat. 2022 Feb;43(2):240-252. doi: 10.1002/humu.24320. Epub 2021 Dec 28. Hum Mutat. 2022. PMID: 34923728 Free PMC article.
References
-
- Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–148. - PubMed
-
- Beisson J, Wright M. Basal body/centriole assembly and continuity. Curr Opin Cell Biol. 2003;15:96–104. - PubMed
-
- Billingsley G, et al. Mutations in chaperonin-like BBS genes are a major contributor to disease development in a multiethnic Bardet-Biedl syndrome patient population. J Med Genet. 2010;47:453–463. - PubMed
-
- Blacque OE, et al. Functional genomics of the cilium, a sensory organelle. Curr Biol. 2005;15:935–941. - PubMed
-
- Braet F, de ZR, Wisse E. Drying cells for SEM, AFM and TEM by hexamethyldisilazane: a study on hepatic endothelial cells. J Microsc. 1997;186:84–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

