Differential recognition of a dileucine-based sorting signal by AP-1 and AP-3 reveals a requirement for both BLOC-1 and AP-3 in delivery of OCA2 to melanosomes
- PMID: 22718909
- PMCID: PMC3418312
- DOI: 10.1091/mbc.E11-06-0509
Differential recognition of a dileucine-based sorting signal by AP-1 and AP-3 reveals a requirement for both BLOC-1 and AP-3 in delivery of OCA2 to melanosomes
Abstract
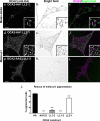
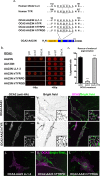
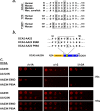
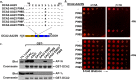
Cell types that generate unique lysosome-related organelles (LROs), such as melanosomes in melanocytes, populate nascent LROs with cargoes that are diverted from endosomes. Cargo sorting toward melanosomes correlates with binding via cytoplasmically exposed sorting signals to either heterotetrameric adaptor AP-1 or AP-3. Some cargoes bind both adaptors, but the relative contribution of each adaptor to cargo recognition and their functional interactions with other effectors during transport to melanosomes are not clear. Here we exploit targeted mutagenesis of the acidic dileucine-based sorting signal in the pigment cell-specific protein OCA2 to dissect the relative roles of AP-1 and AP-3 in transport to melanosomes. We show that binding to AP-1 or AP-3 depends on the primary sequence of the signal and not its position within the cytoplasmic domain. Mutants that preferentially bound either AP-1 or AP-3 each trafficked toward melanosomes and functionally complemented OCA2 deficiency, but AP-3 binding was necessary for steady-state melanosome localization. Unlike tyrosinase, which also engages AP-3 for optimal melanosomal delivery, both AP-1- and AP-3-favoring OCA2 variants required BLOC-1 for melanosomal transport. These data provide evidence for distinct roles of AP-1 and AP-3 in OCA2 transport to melanosomes and indicate that BLOC-1 can cooperate with either adaptor during cargo sorting to LROs.
Figures



Similar articles
-
Functions of adaptor protein (AP)-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes.Mol Biol Cell. 2005 Nov;16(11):5356-72. doi: 10.1091/mbc.e05-07-0626. Epub 2005 Sep 14. Mol Biol Cell. 2005. PMID: 16162817 Free PMC article.
-
Localization to mature melanosomes by virtue of cytoplasmic dileucine motifs is required for human OCA2 function.Mol Biol Cell. 2009 Mar;20(5):1464-77. doi: 10.1091/mbc.e08-07-0710. Epub 2008 Dec 30. Mol Biol Cell. 2009. PMID: 19116314 Free PMC article.
-
BLOC-2, AP-3, and AP-1 proteins function in concert with Rab38 and Rab32 proteins to mediate protein trafficking to lysosome-related organelles.J Biol Chem. 2012 Jun 1;287(23):19550-63. doi: 10.1074/jbc.M112.351908. Epub 2012 Apr 16. J Biol Chem. 2012. PMID: 22511774 Free PMC article.
-
Cell type-specific Rab32 and Rab38 cooperate with the ubiquitous lysosome biogenesis machinery to synthesize specialized lysosome-related organelles.Small GTPases. 2013 Jan-Mar;4(1):16-21. doi: 10.4161/sgtp.22349. Epub 2012 Dec 17. Small GTPases. 2013. PMID: 23247405 Free PMC article. Review.
-
Sorting and targeting of melanosomal membrane proteins: signals, pathways, and mechanisms.Pigment Cell Res. 2000 Jun;13(3):128-34. doi: 10.1034/j.1600-0749.2000.130302.x. Pigment Cell Res. 2000. PMID: 10885669 Review.
Cited by
-
The metabolism of melanin synthesis-From melanocytes to melanoma.Pigment Cell Melanoma Res. 2024 Jul;37(4):438-452. doi: 10.1111/pcmr.13165. Epub 2024 Mar 6. Pigment Cell Melanoma Res. 2024. PMID: 38445351 Free PMC article. Review.
-
BLOC-1 is required for selective membrane protein trafficking from endosomes to primary cilia.J Cell Biol. 2017 Jul 3;216(7):2131-2150. doi: 10.1083/jcb.201611138. Epub 2017 Jun 2. J Cell Biol. 2017. PMID: 28576874 Free PMC article.
-
The AP-3 adaptor complex mediates sorting of yeast and mammalian PQ-loop-family basic amino acid transporters to the vacuolar/lysosomal membrane.Sci Rep. 2015 Nov 18;5:16665. doi: 10.1038/srep16665. Sci Rep. 2015. PMID: 26577948 Free PMC article.
-
Recruitment of septin cytoskeletal proteins by botulinum toxin A protease determines its remarkable stability.J Cell Sci. 2014 Aug 1;127(Pt 15):3294-308. doi: 10.1242/jcs.146324. Epub 2014 Jun 13. J Cell Sci. 2014. PMID: 24928902 Free PMC article.
-
A zinc transporter, transmembrane protein 163, is critical for the biogenesis of platelet dense granules.Blood. 2021 Apr 1;137(13):1804-1817. doi: 10.1182/blood.2020007389. Blood. 2021. PMID: 33513603 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

