Topoisomerase II is required for the production of long Pol II gene transcripts in yeast
- PMID: 22718977
- PMCID: PMC3439932
- DOI: 10.1093/nar/gks626
Topoisomerase II is required for the production of long Pol II gene transcripts in yeast
Abstract
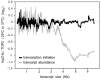
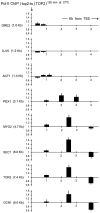
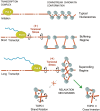
The extent to which the DNA relaxation activities of eukaryotic topoisomerases (topo I and topo II) are redundant during gene transcription is unclear. Although both enzymes can often substitute for each other in vivo, studies in vitro had revealed that the DNA cross-inversion mechanism of topo II relaxes chromatin more efficiently than the DNA strand-rotation mechanism of topo I. Here, we show that the inactivation of topo II in budding yeast produces an abrupt decrease of virtually all polyA+ RNA transcripts of length above ≈ 3 kb, irrespective of their function. This reduction is not related to transcription initiation but to the stall of RNA polymerase II (Pol II) during elongation. This reduction does not occur in topo I mutants; and it is not avoided by overproducing yeast topo I or bacterial topo I, which relaxes (-) DNA supercoils. It is rescued by catalytically active topo II or a GyrBA enzyme, which relaxes (+) DNA supercoils. These findings demonstrate that DNA relaxation activities of topo I and topo II are not interchangeable in vivo. Apparently, only topo II relaxes efficiently the (+) DNA supercoils that stall the advancement of Pol II in long genes. A mechanistic model is proposed.
Figures

References
-
- Roca J. The torsional state of DNA within the chromosome. Chromosoma. 2011;120:323–334. - PubMed
-
- Champoux JJ. DNA topoisomerases: structure, function, and mechanism. Annu. Rev. Biochem. 2001;70:369–413. - PubMed
-
- Wang JC. Cellular roles of DNA topoisomerases: a molecular perspective. Nat. Rev. Mol. Cell Biol. 2002;3:430–440. - PubMed
-
- Schoeffler AJ, Berger JM. DNA topoisomerases: harnessing and constraining energy to govern chromosome topology. Q. Rev. Biophys. 2008;41:41–101. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

