MicroRNA-204 critically regulates carcinogenesis in malignant peripheral nerve sheath tumors
- PMID: 22718995
- PMCID: PMC3408257
- DOI: 10.1093/neuonc/nos124
MicroRNA-204 critically regulates carcinogenesis in malignant peripheral nerve sheath tumors
Abstract
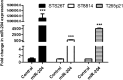
Malignant peripheral nerve sheath tumors (MPNSTs) are highly aggressive soft tissue sarcomas accounting for 3%-10% of all soft tissue sarcomas. Neurofibromatosis type 1 (NF1) is the most important known risk factor. MPNSTs are often diagnosed at an advanced stage when distant metastases have developed. Although surgical resection remains the main treatment for MPNSTs, complete surgical resection is rarely possible. The prognosis for patients with MPNSTs is poor. There is an urgent need for improved therapies. To this end, we investigated whether microRNA (miR), specifically miR-204, might be implicated in MPNSTs because it is located at a cancer-associated genomic region exhibiting high frequency of loss of heterozygosity in tumors. We show that miR-204 expression is downregulated in NF1 and non-NF1 MPNST tumor tissues and in tumor cell lines. Restoring miR-204 expression in MPNST cell lines STS26T (non-NF1), ST88-14 (NF1), and T265p21 (NF1) significantly reduces cellular proliferation, migration, and invasion in vitro. Restoring miR-204 expression in STS26T decreases tumor growth and malignant progression in vivo. We also report that miR-204 inhibits Ras signaling and expression of high mobility group gene A2. These findings support the hypothesis that miR-204 plays critical roles in MPNST development and tumor progression. miR-204 may represent a novel biomarker for diagnosis and a candidate target with which to develop effective therapies for MPNSTs.
Figures





Similar articles
-
Genome-wide transcriptome analyses reveal p53 inactivation mediated loss of miR-34a expression in malignant peripheral nerve sheath tumours.J Pathol. 2010 Jan;220(1):58-70. doi: 10.1002/path.2633. J Pathol. 2010. PMID: 19890883 Free PMC article.
-
MicroRNA-21 correlates with tumorigenesis in malignant peripheral nerve sheath tumor (MPNST) via programmed cell death protein 4 (PDCD4).J Cancer Res Clin Oncol. 2012 Sep;138(9):1501-9. doi: 10.1007/s00432-012-1223-1. Epub 2012 Apr 22. J Cancer Res Clin Oncol. 2012. PMID: 22526161 Free PMC article.
-
Transgenic mice overexpressing neuregulin-1 model neurofibroma-malignant peripheral nerve sheath tumor progression and implicate specific chromosomal copy number variations in tumorigenesis.Am J Pathol. 2013 Mar;182(3):646-67. doi: 10.1016/j.ajpath.2012.11.017. Epub 2013 Jan 13. Am J Pathol. 2013. PMID: 23321323 Free PMC article.
-
Tumor suppressor mutations and growth factor signaling in the pathogenesis of NF1-associated peripheral nerve sheath tumors: II. The role of dysregulated growth factor signaling.J Neuropathol Exp Neurol. 2005 Jan;64(1):1-9. doi: 10.1093/jnen/64.1.1. J Neuropathol Exp Neurol. 2005. PMID: 15715079 Review.
-
Malignant peripheral nerve sheath tumour (MPNST): the clinical implications of cellular signalling pathways.Expert Rev Mol Med. 2009 Oct 19;11:e30. doi: 10.1017/S1462399409001227. Expert Rev Mol Med. 2009. PMID: 19835664 Review.
Cited by
-
Systems Biology Approaches Reveal Potential Phenotype-Modifier Genes in Neurofibromatosis Type 1.Cancers (Basel). 2020 Aug 26;12(9):2416. doi: 10.3390/cancers12092416. Cancers (Basel). 2020. PMID: 32858845 Free PMC article.
-
MicroRNA-204 as an Indicator of Severity of Pulmonary Hypertension in Children with Congenital Heart Disease Complicated with Pulmonary Hypertension.Med Sci Monit. 2019 Dec 30;25:10173-10179. doi: 10.12659/MSM.917662. Med Sci Monit. 2019. PMID: 31887731 Free PMC article.
-
miR-10a and miR-204 as a Potential Prognostic Indicator in Low-Grade Gliomas.Cancer Inform. 2017 Apr 12;16:1176935117702878. doi: 10.1177/1176935117702878. eCollection 2017. Cancer Inform. 2017. PMID: 28469392 Free PMC article.
-
Dysregulation of miRNAs in Soft Tissue Sarcomas.Cells. 2024 Nov 8;13(22):1853. doi: 10.3390/cells13221853. Cells. 2024. PMID: 39594601 Free PMC article. Review.
-
An emerging role for microRNAs in NF1 tumorigenesis.Hum Genomics. 2012 Nov 17;6(1):23. doi: 10.1186/1479-7364-6-23. Hum Genomics. 2012. PMID: 23158014 Free PMC article. Review.
References
-
- Doorn PF, Molenaar WM, Buter J, Hoekstra HJ. Malignant peripheral nerve sheath tumors in patients with and without neurofibromatosis. Eur J Surg Oncol. 1995;21:78–82. - PubMed
-
- Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986;57:2006–2021. - PubMed
-
- King AA, Debaun MR, Riccardi VM, Gutmann DH. Malignant peripheral nerve sheath tumors in neurofibromatosis 1. Am J Med Genet. 2000;93:388–392. - PubMed
-
- Ducatman BS, Scheithauer BW. Postirradiation neurofibrosarcoma. Cancer. 1983;51:1028–1033. - PubMed
-
- Foley KM, Woodruff JM, Ellis FT, Posner JB. Radiation-induced malignant and atypical peripheral nerve sheath tumors. Ann Neurol. 1980;7:311–318. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

