Phosphate/pyrophosphate and MV-related proteins in mineralisation: discoveries from mouse models
- PMID: 22719218
- PMCID: PMC3372882
- DOI: 10.7150/ijbs.4538
Phosphate/pyrophosphate and MV-related proteins in mineralisation: discoveries from mouse models
Abstract
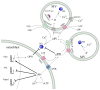
During the process of matrix vesicle (MV)-mediated initiation of mineralisation, chondrocytes and osteoblasts mineralise the extracellular matrix by promoting the seeding of basic calcium phosphate crystals of hydroxyapatite (HA) along the collagen fibrils. This orchestrated process is carefully regulated by the balanced action of propagators and inhibitors of calcification. The primary antagonistic regulators of extracellular matrix mineralisation are phosphate (Pi) and inorganic pyrophosphate (PPi). Studies in mouse models and in humans have established critical roles for Pi/PPi homeostasis in biomineralisation. In this review, we present the regulators of Pi/PPi, as derived from animal models, and discuss their clinical relevance to physiological and pathological mineralisation.
Keywords: MV-related proteins; Matrix vesicles; Mineralisation; OPN.; PPi; Pi.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
References
-
- Anderson HC. Molecular biology of matrix vesicles. Clin Orthop Relat Res. 1995:266–80. - PubMed
-
- Hale JE, Wuthier RE. The mechanism of matrix vesicle formation. Studies on the composition of chondrocyte microvilli and on the effects of microfilament-perturbing agents on cellular vesiculation. J Biol Chem. 1987;262:1916–25. - PubMed
-
- Thouverey C, Strzelecka-Kiliszek A, Balcerzak M. et al. Matrix vesicles originate from apical membrane microvilli of mineralizing osteoblast-like Saos-2 cells. J Cell Biochem. 2009;106:127–38. - PubMed
-
- Thouverey C, Malinowska A, Balcerzak M. et al. Proteomic characterization of biogenesis and functions of matrix vesicles released from mineralizing human osteoblast-like cells. J Proteomics. 2011;74:1123–34. - PubMed
-
- Anderson HC. Matrix vesicles and calcification. Curr Rheumatol Rep. 2003;5:222–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

