Nevirapine- versus lopinavir/ritonavir-based initial therapy for HIV-1 infection among women in Africa: a randomized trial
- PMID: 22719231
- PMCID: PMC3373629
- DOI: 10.1371/journal.pmed.1001236
Nevirapine- versus lopinavir/ritonavir-based initial therapy for HIV-1 infection among women in Africa: a randomized trial
Abstract
Background: Nevirapine (NVP) is widely used in antiretroviral treatment (ART) of HIV-1 globally. The primary objective of the AA5208/OCTANE trial was to compare the efficacy of NVP-based versus lopinavir/ritonavir (LPV/r)-based initial ART.
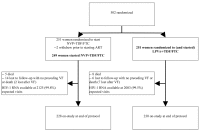
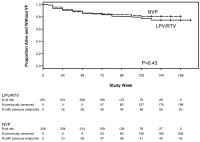
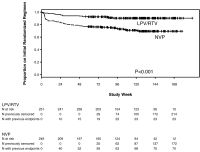
Methods and findings: In seven African countries (Botswana, Kenya, Malawi, South Africa, Uganda, Zambia, and Zimbabwe), 500 antiretroviral-naïve HIV-infected women with CD4<200 cells/mm(3) were enrolled into a two-arm randomized trial to initiate open-label ART with tenofovir (TDF)/emtricitabine (FTC) once/day plus either NVP (n = 249) or LPV/r (n = 251) twice/day, and followed for ≥48 weeks. The primary endpoint was time from randomization to death or confirmed virologic failure ([VF]) (plasma HIV RNA<1 log(10) below baseline 12 weeks after treatment initiation, or ≥400 copies/ml at or after 24 weeks), with comparison between treatments based on hazard ratios (HRs) in intention-to-treat analysis. Equivalence of randomized treatments was defined as finding the 95% CI for HR for virological failure or death in the range 0.5 to 2.0. Baseline characteristics were (median): age = 34 years, CD4 = 121 cells/mm(3), HIV RNA = 5.2 log(10)copies/ml. Median follow-up = 118 weeks; 29 (6%) women were lost to follow-up. 42 women (37 VFs, five deaths; 17%) in the NVP and 50 (43 VFs, seven deaths; 20%) in the LPV/r arm reached the primary endpoint (HR 0.85, 95% CI 0.56-1.29). During initial assigned treatment, 14% and 16% of women receiving NVP and LPV/r experienced grade 3/4 signs/symptoms and 26% and 22% experienced grade 3/4 laboratory abnormalities. However, 35 (14%) women discontinued NVP because of adverse events, most in the first 8 weeks, versus none for LPV/r (p<0.001). VF, death, or permanent treatment discontinuation occurred in 80 (32%) of NVP and 54 (22%) of LPV/r arms (HR = 1.7, 95% CI 1.2-2.4), with the difference primarily due to more treatment discontinuation in the NVP arm. 13 (45%) of 29 women tested in the NVP versus six (15%) of 40 in the LPV/r arm had any drug resistance mutation at time of VF.
Conclusions: Initial ART with NVP+TDF/FTC demonstrated equivalent virologic efficacy but higher rates of treatment discontinuation and new drug resistance compared with LPV/r+TDF/FTC in antiretroviral-naïve women with CD4<200 cells/mm(3).
Trial registration: ClinicalTrials.gov NCT00089505.
Conflict of interest statement
M. Hughes reports receiving fees as a member of the data and safety monitoring boards for Boehringer Ingelheim, Medicines Development, Pfizer, Tibotec, and Virionyx and the receipt by his department of financial support from Schering-Plough and Merck for an annual educational workshop; J. McIntyre, receiving financial support from the Abbott Speakers Bureau; M. Hosseinipour, receiving financial support from Abbott Virology for educational presentations (M. Hosseinipour has given an educational lecture at the 2009 IAS conference and the 2010 INTEREST conference for Abbott virology on the topic of antiretroviral resistance in Malawi); L. Mohapi, receiving reimbursement for travel expenses from Pfizer Laboratories (L. Mohapi received travel, accommodation and registration assistance for the XVII AIDS Conference in Mexico City, August 2008, from Pfizer); J. Mellors, serving on the scientific advisory board for Gilead Sciences, receiving consulting fees from Merck, Idenix Pharmaceuticals, Chimerix, RFS Pharmaceuticals, Panacos Pharmaceuticals, and Abbott Laboratories, receiving grant support from Merck, having stock options in RFS Pharmaceuticals, and receiving reimbursement for travel expenses from Gilead Sciences, Merck, Chimerix, Idenix Pharmaceuticals, RFS Pharmaceuticals, and Panacos Pharmaceuticals; R. Schooley, receiving consulting fees from Glaxo-SmithKline and Abbott Laboratories and reimbursement for travel expenses from Abbott Laboratories (and serves on the scientific advisory board for Gilead Sciences); and J. Currier, receiving consulting fees from GlaxoSmithKline and grant support from Merck and Tibotec. All other authors have declared that no competing interests exist.
Figures
References
-
- WHO. Antiretroviral therapy for HIV infection in adults and adolescents: Recommendations for a public health approach. 2010 Revision. Geneva: World Health Organization; 2010. - PubMed
-
- Rey D, Hoen B, Chavanet P, Schmitt MP, Hoizey G, et al. High rate of early virological failure with the once-daily tenofovir/lamivudine/nevirapine combination in naive HIV-1-infected patients. J Antimicrob Chemother. 2009;63:380–388. - PubMed
-
- Lapadula G, Costarelli S, Quiros-Roldan E, Calabresi A, Izzo I, et al. Risk of early virological failure of once-daily tenofovir-emtricitabine plus twice-daily nevirapine in antiretroviral therapy-naive HIV-infected patients. Clin Infect Dis. 2008;46:1127–1129. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- AI68634/AI/NIAID NIH HHS/United States
- UM1 AI069501/AI/NIAID NIH HHS/United States
- IAAY1AI8374/AI/NIAID NIH HHS/United States
- K24 AI056933/AI/NIAID NIH HHS/United States
- 3U01AI32775-13S5/AI/NIAID NIH HHS/United States
- K24 AI56933/AI/NIAID NIH HHS/United States
- U01 AI069518/AI/NIAID NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- AI-069501/AI/NIAID NIH HHS/United States
- 5U01AI069455-03/AI/NIAID NIH HHS/United States
- UM1 AI069426/AI/NIAID NIH HHS/United States
- U01 AI069453/AI/NIAID NIH HHS/United States
- U01AI069436/AI/NIAID NIH HHS/United States
- 5 U01 AI069518/AI/NIAID NIH HHS/United States
- U01 AI069463/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- U01 AI069501/AI/NIAID NIH HHS/United States
- U01 AI032775/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- AI38838/AI/NIAID NIH HHS/United States
- UM1 AI069453/AI/NIAID NIH HHS/United States
- U01 AI69463-03/AI/NIAID NIH HHS/United States
- 5U01AI069456-03/AI/NIAID NIH HHS/United States
- U01 AI069426/AI/NIAID NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- U01 AI069455/AI/NIAID NIH HHS/United States
- AI69426/AI/NIAID NIH HHS/United States
- AI69453/AI/NIAID NIH HHS/United States
- U01 AI069456/AI/NIAID NIH HHS/United States
- U01AI068636/AI/NIAID NIH HHS/United States
- U01 AI069436/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

